Abstract
Purpose of Review
Breast cancer–related lymphedema (BCRL) is a chronic disease that results from a disruption or obstruction in the lymphatic system and affects 15 in 100 individuals in the USA with newly diagnosed breast cancer. As no curative therapy exists for lymphedema, early detection is crucial in order to reduce the risk of developing late stage symptoms, such as swelling, decreased limb flexibility, disfigurement, and impaired function of the extremity. The objective of this review is to discuss current modalities and devices as well as highlight promising advancements intended to aid in diagnosing secondary lymphedema in breast cancer patients.
Recent Findings
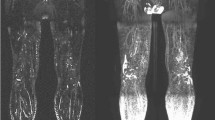
Imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) can offer high resolution of the lymphatics but are expensive and time-consuming. Single photon emission computed tomography (SPECT) is an alternative that reveals organ function as opposed to organ structure. Other imaging methods, such as color duplex ultrasound (CDU), laser scanner 3D (LS3D), and dual-energy X-ray absorptiometry (DXA), are relatively easy to use, reproducible, and fast to perform. However, the disadvantages of these techniques include lower sensitivity and specificity compared with CT and MRI. Of note, direct imaging techniques are highly effective for the diagnosis of lymphedema because they utilize dyes or radiotracers in order to directly visualize lymphatic vessels. Fluorescent microlymphography (FMLG) and near-infrared imaging (NIR) involve injection of fluorescent dyes that can be excited with light. Lymphoscintigraphy has effectively replaced lymphangiography as the method of choice for the diagnosis of lymphedema because it is safer, less invasive, and has no risk of causing an allergic reaction in patients. Novel approaches that are currently in development include bioimpedance spectroscopy, ultra-high-frequency ultrasound systems (UHFUS), and magnetic resonance lymphography (MRL).
Summary
The wide range of diagnostic methods for BCRL exhibit the tradeoff between simplicity and sensitivity; some techniques provide high resolution but are expensive and time consuming. On the other hand, other modalities are easy to use, reliable, and relatively fast in execution yet lack the ability to precisely visualize the lymphatic system. In review of these various techniques, lymphoscintigraphy serves as a clear gold standard for diagnosing secondary lymphedema while more advanced and promising techniques continue to emerge as newer alternatives in clinical practice.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Group USCSW. U.S. Cancer Statistics Data Visualizations Tool, based on November 2018 submission data (1999-2016). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed 6 Aug 2019.
Lawenda B, Mondry T, Johnstone P. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59:8–24. https://doi.org/10.3322/caac.20001.
Mak S, Yeo W, Lee Y, Mo K, Tse K, Tse S, et al. Predictors of lymphedema in patients with breast cancer undergoing axillary lymph node dissection in Hong Kong. Nurs Res. 2008;57:416–25. https://doi.org/10.1097/NNR.0b013e31818c3de2.
McLaughlin S, Bagaria S, Gibson T, Arnold M, Diehl N, Crook J, et al. Trends in risk reduction practices for the prevention of lymphedema in the first 12 months after breast cancer surgery. J Am Coll Surg. 2013;216:380–9. https://doi.org/10.1016/j.jamcollsurg.2012.11.004.
Morrell R, Halyard M, Schild S, Ali M, Gunderson L, Pockaj B. Breast cancer-related lymphedema. Mayo Clin Proc. 2005;80:1480–4. https://doi.org/10.4065/80.11.1480.
• Pamarthi V, Pabon-Ramos W, Marnell V, Hurwitz L. MRI of the central lymphatic system: indications, imaging, technique, and pre-procedural planning. Top Magn Reson Imaging. 2017;26:175–80. https://doi.org/10.1097/RMR.0000000000000130This review describes advances in magnetic resonance (MR) software that allow improved visualization of the lymphatics. In providing helpful visualization of central lymphatic system anatomy and pathology, this technology can be utilized for both lymphedema diagnosis and pre-procedural planning.
Scallan J, Zawieja S, Castorena-Gonzalez J, Davis M. Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol. 2016;594:5749–68. https://doi.org/10.1113/JP272088.
Hancock D, Potezny T, White P. The peripheral lymphatics as an active player in the immune response. J Clin Cell Immunol. 2014;5:268. https://doi.org/10.4172/2155-9899.1000268.
Padera T, Meijer E, Munn L. The lymphatic system in disease processes and cancer progression. Annu Rev Biomed Eng. 2016;18:125–58. https://doi.org/10.1146/annurev-bioeng-112315-031200.
Greene A. History and physical examination. In: Greene A, Slavin S, Brorson H, editors. Lymphedema. Cham: Springer; 2015.
Gerber L. A review of measures of lymphedema. Cancer. 1998;83:2803–4. https://doi.org/10.1002/(sici)1097-0142(19981215)83:12b+<2803::aid-cncr29>3.3.co;2-n.
Lymphology ISo. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46:1–11.
Schook C, Mulliken J, Fishman S, Grant F, Zurakowski D, Greene A. Primary lymphedema: clinical features and management in 138 pediatric patients. Plast Reconstr Surg. 2011;127:2419–31. https://doi.org/10.1097/PRS.0b013e318213a218.
Shin S, Lee W, Park E, Shin C, Chung J, Park J. Comparison of characteristic CT findings of lymphedema, cellulitis, and generalized edema in lower leg swelling. Int J Card Imaging. 2013;29:135–43. https://doi.org/10.1007/s10554-013-0332-5.
Liu N, Wang C, Sun M. Noncontrast three-dimensional magnetic resonance imaging vs lymphoscintigraphy in the evaluation of lymph circulation disorders: a comparative study. J Vasc Surg. 2005;41:69–75. https://doi.org/10.1016/j.jvs.2004.11.013.
Bourgeois P. Combined role of lymphoscintigraphy, x-ray computed tomography, magnetic resonance imaging, and positron emission tomography in the management of lymphedematous disease. In: Lee B, Bergan J, Rockson S, editors. Lymphedema. London: Springer; 2011.
Nishiyama Y, Yamamoto Y, Mori Y, Satoh K, Takashamia H, Ohkawa M, et al. Usefulness of Technetium-99m human serum albumin lymphoscintigraphy in chyluria. Clin Nucl Med. 1998;23:429–31. https://doi.org/10.1097/00003072-199807000-00006.
Cavezzi A. Duplex ultrasonography. In: Lee B, Bergan J, Rockson S, editors. Lymphedema. London: Springer; 2011.
Cammarota T, Pinto F, Magliaro A, Sarno A. Current uses of diagnostic high-frequency US in dermatology. Eur J Radiol. 1998;27:S215–S23. https://doi.org/10.1016/S0720-048X(98)00065-5.
Matter D, Grosshans E, Muller J, Furderer C, Mathelin C, Warter S, et al. Sonographic imaging of lymphatic vessels compared to other methods. J Radiol. 2002;83:599–609.
Suehiro K, Morikage N, Murakami M, Yamashita O, Samura M, Hamano K. Significance of ultrasound examination of skin and subcutaneous tissue in secondary lower extremity lymphedema. Ann Vasc Dis. 2013;6:180–8. https://doi.org/10.3400/avd.oa.12.00102.
Krasnow A, Elgazzar A, Kazem N. Lymphoscintigraphy. In: Elgazzar A, editor. The pathophysiologic basis of nuclear medicine. Berlin, Heidelberg: Springer; 2006.
Ohtake E, Matsui K. Lymphoscintigraphy in patients with lymphedema: a new approach using intradermal injections of technetium-99m human serum albumin. Clin Nucl Med. 1986;11:474–8.
•• O’Donnell T, Rasmussen J, Sevick-Muraca E. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord. 2017;5:261–73. https://doi.org/10.1016/j.jvsv.2016.10.083This review examines new diagnostic modalities for evaluating lymphedema and evaluates the utility of each modality. The strength of the literature in support of each modality offers helpful context for physicians to decide which modality to apply in individual patient cases.
Allegra C, Bartolo M, Carlizza A. Fluorescent microlymphaniography. In: Lee B, Bergan J, Rockson S, editors. Lymphedema. London: Springer; 2011.
Mulasi U, Kuchnia A, Cole A, Earthman C. Bioimpedance at the bedside: current applications, limitations, and opportunitie. Nutr Clin Pract. 2015;30:180–93. https://doi.org/10.1177/0884533614568155.
Cornish B, Chapman M, Hirst C, Mirolo B, Bunce I, Ward L, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2–11.
Czerniec S, Ward L, Lee M, Refshauge K, Beith J, Kilbreath S. Segmental measurement of breast cancer-related arm lymphoedema using perometry and bioimpedance spectroscopy. Support Care Cancer. 2011;19:703–10. https://doi.org/10.1007/s00520-010-0896-8.
Ward L. Bioelectrical impedance analysis: proven utility in lymphedema risk assessment and therapeutic monitoring. Lymphat Res Biol. 2006;4:51–6. https://doi.org/10.1089/lrb.2006.4.51.
Cornish B, Thomas B, Ward L. Improved prediction of extracellular and total body water using impedance loci generated by multiple frequency bioelectrical impedance analysis. Phys Med Biol. 1993;38:337–46. https://doi.org/10.1088/0031-9155/38/3/001.
Cornish B, Bunce I, Ward L, Jones L, Thomas B. Bioelectrical impedance for monitoring the efficacy of lymphoedema treatment programmes. Breast Cancer Res Treat. 1996;38:169–76. https://doi.org/10.1007/BF01806671.
Ward L, Dylke E, Czerniec S, Isenring E, Kilbreath S. Confirmation of the reference impedance ratios used for assessment of breast cancer-related lymphedema by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011;9:47–51. https://doi.org/10.1089/lrb.2010.0014.
• Qin E, Bowen M, James S, Chen W. Multi-segment bioimpedance can assess patients with bilateral lymphedema. J Plast Reconstr Aesthet Surg. 2019. https://doi.org/10.1016/j.bjps.2019.06.041This single institution study examined the role of bioimpedance spectroscopy as a lymphedema diagnostic modality. Single-segment bioimpedance (SSB) was more sensitive than multi-segment impedance (MSB) for diagnosing unilateral lymphedema, while MSB had greater sensitivity and specificity for diagnosing bilateral lymphedema. Moreover, MSB was found to be easier to perform and therefore adapted in the authors’ department practice.
Maus E, Tan I, Rasmussen J, Marshall M, Fife C, Smith L, et al. Near-infrared fluorescence imaging of lymphatics in head and neck lymphedema. Head Neck. 2012;34:448–53. https://doi.org/10.1002/hed.21538.
Rasmussen J, Aldrich M, Tan I, Darne C, Zhu B, O’Donnell TJ, et al. Lymphatic transport in patients with chronic venous insufficiency and venous leg ulcers following sequential pneumatic compression. J Vasc Surg Venous Lymphat Disord. 2016;4:9–17. https://doi.org/10.1016/j.jvsv.2015.06.001.
Zhu B, Rasmussen J, Litorja M, Sevick-Muraca E. Determining the performance of fluorescence molecular imaging devices using traceable working standards with SI units of radiance. IEEE Trans Med Imaging. 2016;35:802–11. https://doi.org/10.1109/TMI.2015.2496898.
Deltombe T, Jamart J, Recloux S, Legrand C, Vandenbroeck N, Theys S, et al. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40:26–34.
Stout Gergich N, Pfalzer L, McGarvey C, Springer B, Gerber L, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–19. https://doi.org/10.1002/cncr.23494.
Cau N, Galli M, Cimolin V, Grossi A, Battarin I, Puleo G, et al. Quantitative comparison between the laser scanner three-dimensional method and the circumferential method for evaluation of arm volume in patients with lymphedema. J Vasc Surg Venous Lymphat Disord. 2017;6:96–103. https://doi.org/10.1016/j.jvsv.2017.08.014.
Cau N, Galli M, Cimolin V, Aranci M, Caraceni A, Balzarini A. Comparative study between circumferential method and laser scanner 3D method for the evaluation of arm volume in healthy subjects. J Vasc Surg: Venous and Lymphat Disord. 2016;4:64–72. https://doi.org/10.1016/j.jvsv.2015.05.005.
Gjorup C, Zerahn B, Juul S, Hendel H, Christensen K, Holmich L. Repeatability of volume and regional body composition measurements of the lower limb using dual-energy x-ray absorptiometry. J Clin Densitom. 2017;20:82–96. https://doi.org/10.1016/j.jocd.2016.08.009.
Gjorup C, Zerahn B, Hendel H. Assessment of volume measurement of breast cancer-related lymphedema by three methods: circumference measurement, water displacement, and dual energy X-ray absorptiometry. Lymphat Res Biol. 2010;8:111–9. https://doi.org/10.1089/lrb.2009.0016.
Hayashi A, Yamamoto T, Yoshimatsu H, Hayashi N, Furuya M, Harima M, et al. Ultrasound visualization of the lymphatic vessels in the lower leg. Microsurgery. 2016;36:397–401. https://doi.org/10.1002/micr.22414.
Hayashi A, Hayashi N, Yoshimatsu H, Yamamoto T. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J Surg Oncol. 2018;117:290–8. https://doi.org/10.1002/jso.24812.
Hayashi A, Giacalone G, Yamamoto T, Belva F, Visconti G, Hayashi N, et al. Ultra high-frequency ultrasonographic imaging with 70 MHz scanner for visualization of the lymphatic vessels. Plast Reconstr Surg Glob Open. 2019;7:e2086. https://doi.org/10.1097/GOX.0000000000002086.
Grassi R, Lagalla R, Rotondo A. Genomics, proteomics, MEMS and SAIF: which role for diagnostic imaging? Radiol Med. 2008;113:775–8. https://doi.org/10.1007/s11547-008-0309-y.
Grassi R, Cavaliere C, Cozzolino S, Mansi L, Cirillo S, Tedeschi G, et al. Small animal imaging facility: new perspectives for the radiologist. Radiol Med. 2009;114:152–67. https://doi.org/10.1007/s11547-008-0352-8.
Lux F, Mignot A, Mowat P, Louis C, Dufort S, Bernhard C, et al. Ultrasmall rigid particles as multimodal probes for medical applications. Angew Chem Int Ed Eng. 2011;50:12299–303. https://doi.org/10.1002/anie.201104104.
Muller A, Fries P, Jelvani B, Lux F, Rube C, Kremp S, et al. Magnetic resonance lymphography at 9.4T using a gadolinium-based nanoparticle in rats: investigations in healthy animals and in a hindlimb lymphedema model. Investig Radiol. 2017;52:725–33. https://doi.org/10.1097/RLI.0000000000000398.
Taradaj J, Rosinczuk J, Dymarek R, Halski T, Schneider W. Comparison of efficacy of the intermittent pneumatic compression with a high- and low-pressure application in reducing the lower limbs phlebolymphedema. Ther Clin Risk Manag. 2015;11:1545–54. https://doi.org/10.2147/TCRM.S92121.
Adams K, Rasmussen J, Darne C, Tan I, Aldrich M, Marshall M, et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Opt Express. 2010;1:114–25. https://doi.org/10.1364/BOE.1.000114.
Brayton K, Hirsch A, O Brien P, Cheville A, Karaca-Mandic P, Rockson S. Lymphedema prevalence and treatment benefits in cancer: impact of a therapeutic intervention on health outcomes and costs. PLoS One. 2014;9:e114597. https://doi.org/10.1371/journal.pone.0114597.
Mayrovitz H, Ryan S, Hartman J. Usability of advanced pneumatic compression to treat cancer-related head and neck lymphedema: a feasibility study. Head Neck. 2018;40:137–43. https://doi.org/10.1002/hed.24995.
Chang C, Cormier J. Lymphedema interventions: exercise, surgery, and compression devices. Semin Oncol Nurs. 2013;29:28–40. https://doi.org/10.1016/j.soncn.2012.11.005.
Melam G, Buragadda S, Alhusaini A, Arora N. Effect of complete decongestive therapy and home program on health- related quality of life in post mastectomy lymphedema patients. BMC Womens Health. 2016;16:23. https://doi.org/10.1186/s12905-016-0303-9.
Szuba A, Achalu R, Rockson S. Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema: a randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer. 2002;95:2260–7. https://doi.org/10.1002/cncr.10976.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hot Topics in Breast Cancer
Rights and permissions
About this article
Cite this article
Raghuram, A.C., Yu, R.P., Sung, C. et al. The Diagnostic Approach to Lymphedema: a Review of Current Modalities and Future Developments. Curr Breast Cancer Rep 11, 365–372 (2019). https://doi.org/10.1007/s12609-019-00341-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-019-00341-3