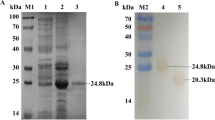
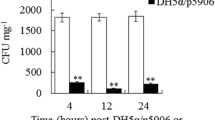
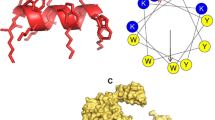
Abstract
Macrobrachium rosenbergii is an economically important source of crustacean seafood worldwide. Vibrio parahaemolyticus is an important aquatic pathogen that causes epidemics of acute hepatopancreatic necrosis in shrimp populations, which results in significant economic losses to aquaculture farmers. To prevent the antibiotics abuse, which has become a serious threat to human health, novel anti-infective strategies are urgently required to control V. parahaemolyticus. Antimicrobial peptides, which exhibit favourable germicidal activity compared to traditional antibiotics, can be used as a key method to prevent and treat bacterial diseases. Herein, an antimicrobial peptide, bomidin, was expressed through genetic engineering technology. The minimum inhibitory concentration (MIC) of bomidin showed a significant inhibitory effect on V. parahaemolyticus that was equivalent to that of ampicillin. Subsequently, the mechanism of action of recombinant bomidin was explored using PNP and ONPG assays to investigate the effects on membrane permeability. These assays indicated that bomidin penetrated the germ membrane and induced the release of cytoplasmic contents and ultimately interacted with DNA to form a bomidin–DNA complex that inhibits bacterial survival. Transmission electron microscopy and scanning electron microscopy revealed that bomidin could cause damage and dysfunction to the cell wall and membrane. Bomidin was nontoxic to mouse red blood cells within a concentration range that was much larger than the MIC. Toxicity assays revealed that 0.02 mg/mL bomidin was safe for use with juvenile freshwater prawns of M. rosenbergii and significantly inhibited the growth of V. parahaemolyticus in cultured water. These results demonstrated that synthetic peptide bomidin had great antibacterial effect against V. parahaemolyticus and therefore a therapeutic potential in aquaculture.








Similar content being viewed by others
Availability of Data and Material
All data generated or analysed in this study are included in this published article.
References
Chen FL, Chou MC, Jiun YL (2020) Feasibility of vaccination against Macrobrachium rosenbergii nodavirus infection in giant freshwater prawn. Fish Shellfish Immunol 104:431–438. https://doi.org/10.1016/j.fsi.2020.06.039
Morris JG Jr, Black RE (1985) Cholera and other vibrioses in the United States. N Engl J Med 312:343–350. https://doi.org/10.1056/nejm198502073120604
He Y, Jin L, Sun F, Hu Q, Chen L (2016) Antibiotic and heavy-metal resistance of Vibrio parahaemolyticus isolated from fresh shrimps in Shanghai fish markets, China. Environ Sci Pollut Res Int 23:15033–15040. https://doi.org/10.1007/s11356-016-6614-4
Su YC, Liu C (2007) Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol 24:549–558. https://doi.org/10.1016/j.fm.2007.01.005
Daniels NA, Mackinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L (2000) Vibrio parahaemolyticus infections in the United States 1973–1998. J Infect Dis 181:1661–1666. https://doi.org/10.1086/315459
Kwon JY, Kim MK, Mereuta L, Seo CH, Luchian T, Park Y (2019) Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express 9:122. https://doi.org/10.1186/s13568-019-0843-0
Silva NC, Sarmento B, Pintado M (2013) The importance of antimicrobial peptides and their potential for therapeutic use in ophthalmology. Int J Antimicrob Agents 41:5–10. https://doi.org/10.1016/j.ijantimicag.2012.07.020
Silva JOP, Appelberg R, Gama FM (2016) Antimicrobial peptides as novel anti-tuberculosis therapeutics. Biotechnol Adv 34:924–940. https://doi.org/10.1016/j.biotechadv.2016.05.007
Pandey BK, Srivastava S, Singh M, Ghosh JK (2011) Inducing toxicity by introducing a leucine-zipper-like motif in frog antimicrobial peptide, magainin 2. Biochem J 436:609–620. https://doi.org/10.1042/bj20110056
Hein-Kristensen L, Knapp KM, Franzyk H, Gram L (2011) Bacterial membrane activity of α-peptide/β-peptoid chimeras: influence of amino acid composition and chain length on the activity against different bacterial strains. BMC Microbiol 11:144. https://doi.org/10.1186/1471-2180-11-144
Liu Y, Knapp KM, Yang L, Molin S, Franzyk H, Folkesson A (2013) High in vitro antimicrobial activity of β-peptoid-peptide hybrid oligomers against planktonic and biofilm cultures of Staphylococcus epidermidis. Int J Antimicrob Agents 41:20. https://doi.org/10.1016/j.ijantimicag.2012.09.014
Hancock RE (2001) Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 1:156–164. https://doi.org/10.1016/S1473-3099(01)00092-5
Ma Z, Wei D, Yan P, Zhu X, Shan A, Bi Z (2015) Characterization of cell selectivity, physiological stability and endotoxin neutralization capabilities of α-helix-based peptide amphiphiles. Biomaterials 52:517–530. https://doi.org/10.1016/j.biomaterials.2015.02.063
Benincasa M, Scocchi M, Pacor S, Tossi A, Nobili D, Basaglia G, Busetti M, Gennaro R (2006) Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J Antimicrob Chemother 58:950–959. https://doi.org/10.1093/jac/dkl382
Wang G, Watson KM, Buckheit RW Jr (2008) Anti-HIV-1 activity of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob Agents Chemother 52:3438–3440. https://doi.org/10.1128/AAC.00452-08
McGwire BS, Olson CL, Tack BF, Engman DM (2003) Killing of African trypanosomes by antimicrobial peptides. J Infect Dis 188:146–152. https://doi.org/10.1086/375747
Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M (1996) Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements of their anti-microbial and cell lytic activities. J Biol Chem 271:28375–28381. https://doi.org/10.1074/jbc.271.45.28375
Gómez EA, Giraldo P, Orduz S (2017) InverPep: a database of invertebrate antimicrobial peptides. J Glob Antimicrob Resist 8:13–17. https://doi.org/10.1016/j.jgar.2016.10.003
Pearson CS, Kloos Z, Murray B, Tabe E, Gupta M, Kwak JH, Karande P, Mcdonough KA, Belfort G (2016) Combined bioinformatic and rational design approach to develop antimicrobial peptides against Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:2757–2764. https://doi.org/10.1128/aac.00940-15
Wang X, Wang G (2016) Insights into antimicrobial peptides from spiders and scorpions. Protein Pept Lett 23:707–721. https://doi.org/10.2174/0929866523666160511151320
Viau C, Sage VL, Ting DK, Gross J, Moual HL (2011) Absence of PmrAB-mediated phosphoethanolamine modifications of Citrobacter rodentium lipopolysaccharide affects outer membrane integrity. J Bacteriol 193:2168–2176. https://doi.org/10.1128/JB.01449-10
Komaniecka I, Zamłyńska K, Zan R, Staszczak M, Pawelec J, Seta I, Choma A (2016) Rhizobium strains differ considerably in outer membrane permeability and polymyxin B resistance. Acta Biochim Pol 63:517–525. https://doi.org/10.18388/abp.2015_1212
Ibrahim HR, Sugimoto Y, Aoki T (2000) Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim Biophys Acta 1523:196–205. https://doi.org/10.1016/s0304-4165(00)00122-7
Jang WS, Kim HK, Lee KY, Kim SA, Han YS, Lee IH (2006) Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. FEBS Lett 580:1490–1496. https://doi.org/10.1016/j.febslet.2006.01.041
Osunde IM, Coyle S, Tidwell J (2004) Acute toxicity of copper to juvenile freshwater prawns. J Appl Aquacult 14:71–79.https://doi.org/10.1300/j028v14n03_06
Helander IM, Mattila-Sandholm T (2000) Fluorometric assessment of gram-negative bacterial permeabilization. J Appl Microbiol 88:213–219. https://doi.org/10.1046/j.1365-2672.2000.00971.x
Eumkeb G, Chukrathok S (2013) Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant Enterobacter cloacae. Phytomedicine 20:262–269. https://doi.org/10.1016/j.phymed.2012.10.008
Kaushal A, Gupta K, Shah R, van Hoek ML (2016) Antimicrobial activity of mosquito cecropin peptides against Francisella. Dev Comp Immunol 63:171–180. https://doi.org/10.1016/j.dci.2016.05.018
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. https://doi.org/10.1038/nrmicro1098
Park J, Kang HK, Choi MC, Chae JD, Son BK, Chong YP, Seo CH, Park Y (2018) Antibacterial activity and mechanism of action of analogues derived from the antimicrobial peptide mBjAMP1 isolated from Branchiostoma japonicum. J Antimicrob Chemother 73:2054–2063. https://doi.org/10.1093/jac/dky144
Nam J, Yun H, Rajasekaran G, Kumar SD, Kim JI, Min HJ, Shin SY, Lee CW (2018) Structural and functional assessment of mBjAMP1, an antimicrobial peptide from Branchiostoma japonicum, revealed a novel α-hairpinin-like scaffold with membrane permeable and DNA binding activity. J Med Chem 61:11101–11113. https://doi.org/10.1021/acs.jmedchem.8b01135
Cheng AC, Lin HL, Shiu YL, Tyan YC, Liu CH (2017) Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture. Fish Shellfish Immunol 67:270–279. https://doi.org/10.1016/j.fsi.2017.06.006
Lee BC, Hung CW, Lin CY, Shih CH, Tsai HJ (2019) Oral administration of transgenic biosafe microorganism containing antimicrobial peptide enhances the survival of tilapia fry infected bacterial pathogen. Fish Shellfish Immunol 95:606–616. https://doi.org/10.1016/j.fsi.2019.10.052
Acknowledgements
We would like to thank the support staff for their immense.
Funding
This study was funded by the Jiangsu Agricultural Independent Innovation Project (SCX(18)2011), National Key Research and Development Program of China (2017YFF0208600), National “Youth Top-notch Talent” Support Program (W0270187), Introduction of Nanjing Agricultural University Scientific Research Grants Project (804121), Central Guidance for Local Science and Technology Development (No. YDZX20173100004528), Science and Technology Joint Project of the Yangzte River Delta (No. 17395810102) and Jiangsu Collaborative Innovation Center of Meat Production and Processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Yu, Y., Lian, L. et al. Functional Mechanism of Antimicrobial Peptide Bomidin and Its Safety for Macrobrachium rosenbergii. Probiotics & Antimicro. Prot. 14, 169–179 (2022). https://doi.org/10.1007/s12602-021-09857-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09857-6