Abstract
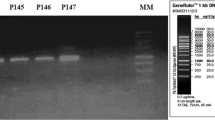
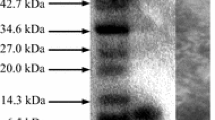
A total of 145 lactic acid bacteria isolated from a variety of Turkish red wines during malolactic fermentation were screened to find bacteriocin-producing strains. Among them, 14 isolates of Enterococcus faecium were identified to produce bacteriocins. PCR screening revealed that some isolates harbored entA and entB genes while some harbored entA, entB and entP genes. An isolate designated as Ent. faecium H46 was selected to characterize its bacteriocins. The bacteriocins were purified to homogeneity from culture supernatant by Amberlite XAD-16, cation-exchange and reverse-phase chromatography. MALDI-TOF mass spectrometry analysis identified the bacteriocins as enterocin A and enterocin B. The presence of Ent. faecium is noteworthy since it is not associated with wine fermentation. However, it has been reported as an important wine spoilage organism due to its potential to produce tyramine. Although species of Enterococcus is not known as wine bacteria, contamination by Ent. faecium may arise from grapes or wineries equipments used for wine production.





Similar content being viewed by others
References
Jack RW, Tagg JR, Ray B (1995) Bacteriocins of gram-positive bacteria. Microbiol Rev 59:171–200
Perez RH, Zendo T, Sonomoto K (2014) Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact 13(Suppl 1):S3
Radler F (1990) Possible use of nisin in winemaking. I. Action of nisin against lactic acid bacteria and wine yeasts in solid and liquid media. Am J Enol Vitic 41:1–6
Yurdugül S, Bozoğlu F (2002) Studies on an inhibitor produced by LAB of wines on the control of malolactic fermentation. Eur Food Res Technol 215:38–41
Bauer R, Nel HA, Dicks LMT (2003) Pediocin PD-1 as a method to control growth of Oenococcus oeni in wine. Am J Enol Vitic 54:86–91
Rojo-Bezares B, Sáenz Y, Zarazaga M, Torres C, Ruiz-Larrea F (2007) Antimicrobial activity of nisin against Oenococcus oeni and other wine bacteria. Int J Food Microbiol 116:32–36
Díez L, Rojo-Bezares B, Zarazaga M, Rodríguez JM, Torres C, Ruiz-Larrea F (2012) Antimicrobial activity of pediocin PA-1 against Oenococcus oeni and other wine bacteria. Food Microbiol 31:167–172
Navarro L, Zarazaga M, Saenz JS, Ruiz-Larrea F, Torres C (2000) Bacteriocin production by lactic acid bacteria isolated from Rioja red wines. J Appl Microbiol 88:44–51
Holo H, Jeknic Z, Daeschel M, Stevanovic S, Nes IF (2001) PlantaricinW from Lactobacillus plantarum belongs to a new family of two-peptide lantibiotics. Microbiology 147:643–651
Strasser de Saad AM, Manca de Nadra MC (1993) Characterization of bacteriocin produced by Pediococcus pentosaceus from wine. J Appl Bacteriol 74:406–410
Ndlovu B, Schoeman H, Franz CM, du Toit M (2015) Screening, identification and characterization of bacteriocins produced by wine-isolated LAB strains. J Appl Microbiol 118:1007–1022
Van Vuuren HJJ, Dicks LMT (1993) Leuconostoc oenos: a review. Am J Enol Vitic 44:99–112
Lonvaud-Funel A (1999) Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Van Leeuwenhoek 76:317–331
Marcobal A, de las Rivas B, García-Moruno E, Muñoz R (2004) The tyrosine decarboxylation test does not differentiate Enterococcus faecalis from Enterococcus faecium. Syst Appl Microbiol 27:423–426
Renouf V, Claisse O, Lonvaud-Funel A (2005) Understanding the microbial ecosystem on the grape berry surface through numeration and identification of yeast and bacteria. Aust J Grape Wine Res 11:316–327
Bae S, Fleet GH, Heard GM (2006) Lactic acid bacteria associated with wine grapes from several Australian vineyards. J Appl Microbiol 100:712–727
Capozzi V, Ladero V, Beneduce L, Fernández M, Alvarez MA, Benoit B, Laurent B, Grieco F, Spano G (2011) Isolation and characterization of tyramine-producing Enterococcus faecium strains from red wine. Food Microbiol 28:434–439
Kačániová M, Hleba L, Pochop J, Kádasi-Horáková M, Fikselová M, Rovná K (2012) Determination of wine microbiota using classical method, polymerase chain method and Step One Real-Time PCR during fermentation process. J Environ Sci Health B 47:571–578
Liesack W, Weyland H, Stackebrandt E (1991) Potential risks of gene amplification by PCR as determined by 16S rRNA analysis of a mixed-culture of obligately barophilic bacteria. Microb Ecol 21:188–201
Shigematsu T, Hayashi M, Kikuchi I, Ueno S, Masaki H, Fujii T (2009) A culture-dependent bacterial community structure analysis based on liquid cultivation and its application to a marine environment. FEMS Microbiol Lett 293:240–247
Lee HJ, Joo CS, Park SH et al (1999) Purification and characterization of a bacteriocin produced by Lactococcus lactis subsp. lactis H-559 isolated from kimchi. J Biosci Bioeng 88:153–159
Batdorj B, Dalgalarrondo M, Choiset Y, Pedroche J, Métro F, Prévost H, Chobert JM, Haertlé T (2006) Purification and characterization of two bacteriocins produced by lactic acid bacteria isolated from Mongolian airag. J Appl Microbiol 101:837–848
Du Toit M, Franz C, Dicks LMT, Holzapfel WH (2000) Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J Appl Microbiol 88:482–494
De Vuyst L, Foulquie Moreno MR, Revets H (2003) Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol 84:299–318
Booth MC, Bogie CP, Sahl HG, Siezen RL, Hatter KL, Gilmore MS (1996) Structural analysis and proteolytic activation of Enterococcus faecalis cytolysin, a novel lantibiotic. Mol Microbiol 21:1175–1184
Shin MS, Han SK, Ji AR, Kim KS, Lee WK (2008) Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J Appl Microbiol 105:2203–2212
Bhunia AK, Johnson MC, Ray B (1988) Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol 65:261–268
Dündar H, Atakay M, Çelikbıçak Ö, Salih B, Bozoglu F (2015) Comparison of two methods for purification enterocin B produced by Enterococcus faecium W3. Prep Biochem Biotechnol 45:796–809
Aymerich T, Holo H, Håvarstein LS, Hugas M, Garriga M, Nes IF (1996) Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol 62:1676–1682
Floriano B, Ruiz-Barba JL, Jiménez-Díaz R (1998) Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl Environ Microbiol 64:4883–4890
Cintas LM, Casaus P, Herranz C, Håvarstein LS, Holo H, Hernández PE, Nes IF (2000) Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J Bacteriol 182:6806–6814
De Kwaadsteniet M, Todorov SD, Knoetze H, Dicks LMT (2005) Characterization of a 3944 Da bacteriocin, produced by Enterococcus mundtii ST15, with activity against Gram-positive and Gram-negative bacteria. Int J Food Microbiol 105:433–444
Zendo T, Eungruttanagorn N, Fujioka S, Tashiro Y, Nomura K, Sera Y, Kobayashi G, Nakayama J, Ishizaki A, Sonomoto K (2005) Identification and production of a bacteriocin from Enterococcus mundtii QU 2 isolated from soybean. J Appl Microbiol 99:1181–1190
Hu CB, Zendo T, Nakayama J, Sonomoto K (2008) Description of durancin TW-49 M, a novel enterocin B-homologous bacteriocin in carrot-isolated Enterococcus durans QU 49. J Appl Microbiol 105:681–690
Ishibashi N, Himeno K, Fujita K, Masuda Y, Perez RH, Zendo T, Wilaipun P, Leelawatcharamas V, Nakayama J, Sonomoto K (2012) Purification and characterization of multiple bacteriocins and an inducing peptide produced by Enterococcus faecium NKR-5-3 from Thai fermented fish. Biosci Biotechnol Biochem 76:947–953
Gaaloul N, ben Braiek O, Hani K, Volski A (2014) Isolation and characterization of large spectrum and multiple bacteriocin-producing Enterococcus faecium strain from raw bovine milk. J Appl Microbiol 118:343–355
Strompfova V, Laukova A, Simonova M, Marcinakova M (2008) Occurrence of the structural enterocin A, P, B, L50B genes in enterococci of different origin. Vet Microbiol 132:293–301
Aguilar-Galvez A, Dubois-Dauphin R, Campos D, Thonart P (2011) Genetic determination and localization of multiple bacteriocins produced by Enterococcus faecium CWBI-B1430 and Enterococcus mundtii CWBI-B1431. Food Sci Biotechnol 20:289–296
Hu CB, Malaphan W, Zendo T, Nakayama J, Sonomoto K (2010) Enterocin X, a novel two-peptide bacteriocin from Enterococcus faecium KU-B5, has an antibacterial spectrum entirely different from those of its component peptides. Appl Environ Microbiol 76:4542–4545
Van Belkum MJ, Kok K, Venema G, Holo H, Nes IF, Konings WN, Abee T (1991) The bacteriocin lactococcin A specifically increases the permeability of lactococcal cytoplasme membranes in a voltage-independent, protein-mediated manner. J Bacteriol 173:7934–7941
Georgalaki MD, Van den Berghe E, Kritikos D, Devreese B, Van Beeumen J, Kalantzopoulos G, De Vuyst L, Tsakalidou E (2002) Macedocin, a food-grade lantibiotic produced by Streptococcus macedonicus ACADC 198. Appl Environ Microbiol 68:5891–5903
Moreno MRF, Callewaert R, Devreese B, Van Beeumen J, De Vuyst L (2003) Isolation abd biochemical characterization of enterocins produced by enterococci from different sources. J Appl Microbiol 94:214–229
Nes IF, Diep DB, Ike Y (2014) Enterococcal bacteriocins and antimicrobial proteins that contribute to niche control. In: Gilmore MS, Clewell DB, Ike Y, Shankar N (eds) Enterococci: from commensals to leading causes of drug resistant infection [Internet]. Massachusetts Eye and Ear Infirmary, Boston
Walling E, Gindreau E, Lonvaud-Funel A (2005) A putative glucan synthase gene dps producing Pediococcus damnosus and Oenococcus oeni strains isolated from wine and cider. Int J Food Microbiol 98:53–62
Casaus P, Nilsen T, Cintas LM, Nes IF, Hernández PE, Holo H (1997) Enterocin B, a new bacteriocin from Enterococcus faecium TI36 which can act synergistically with enterocin A. Microbiology 143:2287–2294
Rehaiem A, Martίnez B, Manai M, Rodrίguez A (2010) Production of enterocin A by Enterococcus faecium MMRA isolated from ‘Rayeb’, a traditional Tunisian dairy beverage. J Appl Microbiol 108:1685–1693
Schoeman H, Vivier MA, Du Toit M, Dicks LMT, Pretorius IS (1999) The development of bacterial yeast strains by expressing the Pediococcus acidilactici pediocin gene (pedA) in Saccharomyces cerevisiae. Yeast 15:647–656
Van Reenen CA, Chikindas ML, Van Zyl WH, Dicks LMT (2002) Characterization and heterologous expression of a class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum 423, in Saccharomyces cerevisiae. Int J Food Microbiol 81:29–40
Martínez JM, Kok J, Sanders JW, Hernández PE (2000) Heterologous co-production of enterocin A and pediocin PA-1 by Lactococcus lactis: detection by specific peptide-directed antibodies. Appl Environ Microbiol 66:3543–3549
Jiménez JJ, Borrero J, Gútiez L, Arbulu S, Herranz C, Cintas LM, Hernández PE (2014) Use of synthetic genes for cloning, production and functional expression of the bacteriocins enterocin A and bacteriocin E 50–52 by Pichia pastoris and Kluyveromyces lactis. Mol Biotechnol 56:571–583
Tominaga T, Hatakeyama Y (2007) Development of innovative pediocin PA-1 by DNA shuffling among class IIa bacteriocins. Appl Environ Microbiol 73:5292–5299
Aymerich MT, Garriga M, Ylla J, Vallier J, Monfort JM, Hugas M (2000) Application of enterocins as biopreservatives against Listeria innocua in meat products. J Food Prot 63:721–726
Aymerich MT, Garriga M, Costa S, Monfort JM, Hugas M (2002) Prevention of ropiness in cooked pork by bacteriocinogenic cultures. Int Dairy J 12:239–246
Acknowledgments
The author would like to thank Mass Spectrometry Laboratory of Hacettepe University Department of Chemistry (Ankara, Turkey) for the collection of mass spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Dündar, H. Bacteriocinogenic Potential of Enterococcus faecium Isolated from Wine. Probiotics & Antimicro. Prot. 8, 150–160 (2016). https://doi.org/10.1007/s12602-016-9222-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9222-1