Abstract
In recent years, smart materials have piqued the interest of scientists and physicians in the biomedical community owing to their ability to modify their properties in response to an external stimulation or changes in their surroundings. Biocompatible piezoelectric materials are an interesting group of smart materials due to their ability to produce electrical charges without an external power source. Electric signals produced by piezoelectric scaffolds can renew and regenerate tissues through special pathways like that found in the extracellular matrix. This review summarizes the piezoelectric phenomenon, piezoelectric effects generated within biological tissues, piezoelectric biomaterials, and their applications in tissue engineering and their use as biosensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many decades, a major focus of the research in biomaterial field has been concerned with designing biocompatible materials capable of interacting with the biological system—so-called bioactive materials (Enderle 2005). Nowadays, bio-smart materials which are able to respond to external triggers and mimicking the natural biological tissue have attracted remarkable attention (Binyamin et al. 2006, Höcker and Klee 1996). Bio-smart materials (also called reactive, responsive materials or intelligent functional materials) (Ebara et al. 2014) designate a special quality in which the material plays an active role with the “smartness” determined by its inherent ability to sense, diagnose, and respond to outside stimuli (Roy et al. 2010; Gao et al. 2019). Examples of such external stimuli include changes in temperature (Fairman and Åkerfeldt 2005), pH, specific chemicals, and electric or magnetic fields (Anju et al. 2021) with all of these stimuli able to induce some particular change in the characteristics of the smart material in a controlled manner (Jacob et al. 2018). Because of the various possibilities for using smart materials as candidates for producing the next generation of biomedical devices, temporary implants, and drug delivery vehicles, smart materials have piqued the curiosity of both scientists and clinicians (Fernandes et al. 2019).
A range of different types of smart materials are currently available (Wadley 1996; Tzou et al. 2004), with new ones being developed on a daily basis (Akhras 2000; Nerkar et al. 2022). Notable among these new developments are piezoelectric materials (Vijaya 2012), pyroelectric materials (Tzou et al. 2004), shape memory materials (Otsuka and Wayman 1999), chromoactive materials (García Huete 2017), magneto-rheological materials (Ahamed et al. 2018), photoactive materials (Marshall and Dimova-Malinovska, 2002), and ferroelectric materials (Cordero‐Edwards et al. 2017).
Some applications of smart materials in regenerative medicine include tissue engineering (Wang 2017b2016), drug delivery (Sponchioni et al. 2019), diagnosis (Guo et al. 2020), biomedical devices like artificial muscles (Shahinpoor et al. 2007), super-elastic self-expanding nitinol stents (Duerig et al. 2003), clippers and nitinol staples (Chaudhari et al. 2021), materials used in orthodontics (McCabe et al. 2011), the Simon filter used in treatment of cardiovascular disease (Petrini and Migliavacca 2011), and special materials used to create shape memory staples for orthopedics (Seil and Webster 2008).
Piezoelectric phenomenon
Piezoelectricity, also called pressing electricity or the piezoelectric effect, is an unusual property of some dielectric materials that leads them to respond to mechanical stimulus by creating an electrical charge (Fig. 1) (Smith and Kar-Narayan 2022; Arnau and Soares 2009). This process is reversible, which means that the materials can be induced to mechanically deform (leading to a change in their dimensions) by applying an electric field (Ciofani and Menciassi 2012). Bio-piezoelectricity refers to the widespread existence of piezoelectric phenomena within a variety of biological systems and molecules (Stapleton et al. 2016)(Lay et al. 2021).
History of piezoelectricity
Piezoelectricity was first discovered in 1880 by the brothers Jacques and Pierre Curie (Koptsik and Rez 1981). The brothers found that, when stress was applied to some crystals, such as quartz, Rochelle salt, tourmaline, topaz, and sugar cane, electrical charges were generated at their surface and the voltage was found to be equivalent to the applied stress (Thomas et al. 2018). Lippmann (1881) made an important contribution to piezoelectricity by predicting the converse piezoelectric effect on the basis of the arguments made using basic thermodynamic principles (Erhart and Přívratská 2010), a prediction which was later confirmed experimentally by the Curie brothers (Gautschi 2002).
For the next few decades, piezoelectricity remained a laboratory curiosity, something to be tested as more research was done to uncover the piezoelectric effect’s enormous potential. The launch of the first practical application for piezoelectric devices, the sonar device, coincided with the outbreak of World War II (Szabo 2004). The early application of piezoelectricity in sonar sparked a renewed worldwide interest in piezoelectric devices and new piezoelectric materials and applications for those materials were studied and developed throughout the next few decades (Main et al. 1995).
Mechanism of piezoelectricity
Piezoelectricity is an interaction between electrical and mechanical systems within a non-centrosymmetric structural arrangement (Covaci and Gontean 2020). The piezoelectric effect, as depicted in Fig. 2, involves the generation of an electric dipole in a material as a result of an applied force. Before applying an external force to the material, the net dipole effected by the structural unit is zero (which can be thought of as the equal positive and negative aspects of a dipole overlapping) resulting in an electrically neutral arrangement (Park et al. 2020). However, if an external mechanical stress is applied, the basic structural arrangement deforms, producing a net dipole, i.e., the separation of the molecule’s positive and negative centers. As a result, the electrons within the material re-equilibrate and fixed charges emerge on opposing surfaces of the material with the material becoming electrically polarized (Starr and Wang 2015).
Piezoelectric material. The figure represents a 2D lattice. A unit cell is shown outlined with dashed lines. At the left figure, without any external stress, the centroid of positive and negative charges coincide and marked by a black dot. When the material is compressed (right figure), the distance between the atoms remains the same, which is only possible by expanding the material horizontally. This in turn moves the positive and negative charges denoted by a star (*) apart, and their centroid no longer coincide, but are shown by blue and red dots, creating an electric dipole.
Piezoelectric materials and their characteristic parameters
Piezoelectrics are officially defined as a category of dielectric materials that can be polarized under the influence of an electric field and also mechanical stress (Dineva et al. 2014; Ye 2008). Piezoelectricity is a linear electromechanical interaction between the electrical and the mechanical states. The piezoelectric coefficient d is the constant for such a linearly proportional relation. Piezoelectric coefficient d is a third-rank tensor coupling the first rank tensor (electric displacement or field) and the second rank tensor (stress or strain). Therefore, the piezoelectric equations may be written in the form
where Dk is the electric displacement (C/m2), and Tij is the stress component (N/m2). Sij is the strain component, Ei is the electric field component (V/m), and dkij or dkij* is the component of the piezoelectric charge or strain constant, (i, j, k = 1, 2, 3).
From the above tensor description of the coupled strain charge relations, we place our focus on the following useful parameters when evaluating the performance of piezoelectric materials.
(i) Piezoelectric coefficient (dxy), which represents the charge generated per unit of applied force or the deflection per unit of electrical voltage applied (Tichý et al. 2010). Due to its intrinsic vectorial nature, the piezoelectric coefficient is usually written as dxy, with x and y indicating the direction of the electric field and stress or strain, respectively (Soin et al. 2016). In the literature, the longitudinal piezoelectric coefficient (d33), the transverse piezoelectric coefficient (d31), and the tangential piezoelectric coefficient (d15) are commonly reported (Miao and Li 2015).
(ii) The electromechanical coupling coefficient (K), which represents the ratio of electrical and mechanical energies associated with carrying out the piezoelectric transformation (Eq. 3):
(iii) Mechanical quality factor: The mechanical Qm is the ratio of the reactance to the resistance in the series equivalent circuit representing the piezoelectric resonator, which is related to the sharpness of the resonance frequency (Li 2020; Kim 2013). The mechanical Qm can be calculated using the equation:
where f r is the resonance frequency, and f1 and f2 are frequencies at 3 dB of the maximum admittance.
These extraordinary characteristics are exhibited by only a few dielectric materials and indeed piezoelectric materials can be classified into four general classes: crystals, piezoelectric polymers, piezoelectric composites, piezoelectric ceramics (Fig. 3).

Reproduced with permission from Kapat et al. (2020)
Piezoelectric charge constants of some biomaterials. Lead zirconate titanate PZT: Pb(Zr,Ti)O3, Barium titanate BT: BaTiO3, Lead titanate PT: PbTiO3, BN: boron nitride (BN), GaN: gallium nitride, LN: LiNbO3 Lithium niobate, LNKN: Li(Na,K)NbO3 Lithium sodium potassium niobate, KNN: (K,Na)NbO3 potassium sodium niobate, PMN: PbMgNbO3 lead magnesium niobate, BNT: (Bi,Na) TiO3 bismuth sodium titanium oxide, ZnO: zinc oxide, HA: hydroxyapatite, PLLA: poly(l-lactic acid), PVDF: poly(vinylidene fluoride), P(VDF-TrFE): poly(vinylidene fluoride-trifluoro ethylene), PHB: polyhydroxybutyrate, FF-PNT: diphenylalanine-based peptide nanotubes.
Piezoelectric crystals
Crystals are defined as a form of matter in which the atoms, molecules, or ions are arranged in a highly ordered three-dimensional lattice (Guinier 1994). Twenty-one of the 32 crystal classes are non-centrosymmetric, meaning they lack a center of symmetry and can consequently show piezoelectric effects (Wang and Liu 2012). Crystals were the first substance used in the early piezoelectricity research; Rochelle salt crystal was originally produced in 1655 (Cross and Newnham 1987), but it was Valasek who established its ferroelectricity and consequent piezoelectric nature demonstrating a substantial piezoelectric effect (Zhang et al. 2018). The piezoelectric effect was later identified and measured in crystals of potassium dihydrogen phosphate (KDP) and ammonium dihydrogen phosphate (ADP) in the early 1940s (Mason 1946). The ADP crystals were then utilized in high-power acoustic transducers (Zhang et al. 2018).
Perfect crystals featuring a regularly and consistently arranged single asymmetric unit such as SiO2, LiTaO3, LiNbO3, and La3Ga5SiO14 yield a stable and consistent piezoelectric effect (Gonzalez 2016). However, less regularly arranged crystal structures known as polycrystals, such as BaTiO3, Pb[Zr.Ti]O3, and PbTiO3, yield a diverse piezoelectric response (Kholkin et al. 2008). Due to this reason, highly regular artificial crystals have an advantage over their naturally sourced equivalents (Uchino 2017) and due to this reason nearly all modern piezoelectric devices now are made from artificial crystals—with artificial quartz crystals being the most commonly employed (Saigusa2017; Tanaka 1982).
Piezoelectric polymers (piezopolymers)
Polymers are a class of natural or synthetic substances composed of very large molecules, called macromolecules, that are multiples of simpler chemical units called monomers (Tauer 2007). Piezoelectric polymers represent attractive biomaterials because of the low dielectric constant, great mechanical flexibility, light weight, and simplicity of manufacturing (Thuau et al. 2016, Sappati and Bhadra 2018). The industrial processing parameters of such piezopolymers can be adjusted to achieve a desired level of electromechanical coupling efficiency and optical transparency (Dong et al. 2020). Most piezoelectric polymers are biocompatible and exhibit little to no toxicity (Maiti et al. 2019) while some are also biodegradable (Curry et al. 2018). These advantages make these polymers auspicious candidates for the manufacturing of medical devices (Xu et al. 2021b). Examples of piezoelectric polymers used in industry and research include polyvinyl chloride, polyacrylonitrile, poly-3-hydroxybutyrate-3-hydroxyvalerate, poly-β-hydroxybutyrate, poly-l-lactic acid, poly-β-hydroxybutyrate, and odd-numbered nylon (e.g., nylon-11) (Murayama and Obara 1983; Xu et al. 2021a).
Polyvinylidene fluoride (PVDF), one of the most famous piezoelectric polymers, was discovered by Japanese scientists in 1969 (Fukada 2000). PVDF piezoelectric film exhibits strong piezoelectric properties (Broadhurst et al. 1978) and due to this reason piezoelectric PVDF film has evolved into a standard type of piezoelectric sensing material with exceptional performance (Hu et al. 2018). PVDF film possesses low acoustic impedance, high piezoelectric constant, high dielectric strength, and spectacular mechanical characteristics (Sappati and Bhadra 2018). PVDF piezoelectric film sensors have a wide range of applications in biosensors and other domains (Shang et al. 2019). The piezoelectric effect also has been demonstrated in some biopolymers such as cellulose, collagen, chitosan, and chitin (Richter et al. 2008, Poillot et al. 2021). Typically piezoelectric polymers exhibit a low piezoelectric coefficient leading to lower charge generation (Maiti et al. 2019).
Piezoelectric composites (piezocomposites/piezonanocomposites)
To overcome the observed problem of piezopolymers exhibiting low piezoelectric coefficients, they can be combined with high piezoelectric coefficient inorganic materials to form piezocomposites, also called piezonanocomposites (Kapat et al. 2020). Piezoelectric composites have improved piezoelectric characteristics in comparison to non-composite piezopolymers (Akdogan et al. 2005). Piezoelectric composites, as compared to individual piezoelectric constituents, can overcome the temperature boundaries of piezopolymers and the inherent brittleness of inorganic bio-piezomaterials while also allowing for large-area fabrication (Xu et al. 2021b).
Tang et al. prepared a novel bio-piezoelectric composite to be used as bone cement by incorporating barium titanate (BaTiO3 [BT]) particles into the biocompatible polymer polymethyl methacrylate (Tang et al. 2020). Results showed that BaTiO3 [BT] particles improved the osteoinductivity of the cement. A piezoelectric coefficient comparable with that of human bones was obtained with low BT content cement by adding graphene which increases the conductivity and dielectric constant. Such types of bio-piezoelectric composite promoted cell proliferation with the cell count increased with increasing the piezoelectric coefficient.
Lee et al. fabricated a piezoelectric composite from the conventional piezoelectric polymers polyvinylidene fluoride and silk fibroin by using an electrospinning technique (Lee et al. 2021). The fabricated composites were shown to be effective in producing a bio-piezoelectric field as they can keep piezoelectricity while overcoming inefficient physical properties associated with a pure PVDF electrospun mat.
Silva et al. studied the piezoelectric and dielectric properties of collagen-hydroxyapatite composites (Silva et al. 2002). The results revealed that the collagen composites incorporated with nanocrystalline HA presented an improved result (d14 = 0.040 pC/N) compared to the composites with the commercial HA (d14 = 0.012 pC/N).
Piezoelectric ceramics (piezoceramics)
A ceramic is an inorganic non-metallic solid made up of either metal or non-metal compounds that have been shaped and then hardened by heating to high temperatures (Nandhini et al. 2021). Many piezoceramics materials with high piezoelectric coefficient are available (Panda and Sahoo 2015). The first of these that we will discuss is barium titanate (BaTiO3) which was the first piezoelectric ceramic discovered (Vijatović et al. 2008b) and has been found suitable for many applications due to its exceptional piezoelectric and dielectric properties. It can be prepared using several methods depending on the desired application (Aaron et al. 2004, Vijatović et al. 2008a).
Another common piezoceramic, boron nitride nanotubes (BNNT), possesses a high piezoelectric response and has been recently utilized for PC12 neuronal-like cell stimulation as nanotransducer causing improved neurite outgrowth (Ciofani et al. 2011). Zinc oxide (ZnO), a piezoelectric and semiconducting oxide, has become one of the most important materials in nanotechnology (Wang et al. 2004; Wang et al. 2009). It has been utilized in a number of ceramic nanostructures such as nanowires, nanobelts, nanorings, nanosprings, or nanobows (Wang and Song 2006, Nour et al. 2017).
Lead zirconate titanate (PZT) has a very high piezoelectric constant (200–350) pC/N (Gross et al. 2003). Nanostructured PZT in the form of nanoribbons or nanowire form has been developed and applied in the construction of biomedical and energy harvesting devices (Qi and McAlpine 2010). A lot of methods have been developed to prepare PZT ceramics, such as sol–gel techniques (Jacob et al. 2003), the conventional mixed oxides (Xue et al. 1999a), chemical coprecipitation (Goel and Yadav 2005), and mechanochemical synthesis (Xue et al. 1999b) (Branković et al. 2003). However, PZT ceramics have limited applications in tissue engineering because of their cytotoxicity. As a result, lead-free piezoceramics have been developed to lower concerns about toxic environmental exposure from lead (Bell and Deubzer 2018). Lithium sodium potassium niobate (LNKN) and potassium sodium niobate (KNN) are examples recently developed to lower the toxicity of piezoceramics (Rödel et al. 2009). Figure 3 represents piezoelectric charge constants of some piezoelectric biomaterials.
Piezoelectricity within human body (Table 1)
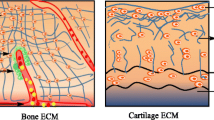
In biological systems, there is much growing evidence to suggest that surface charges and electrostatic interactions play significant roles in regulating the functions of cells and their individual components (Honig and Nicholls 1995), such as in ion channels (Finazzi et al. 2015), cardiac and muscular contraction (Iwazumi and Noble 1989), protein folding (Makhatadze 2017), charged biomolecule interaction (Leung et al. 2009), and pH-dependent processes (Genet et al. 2000). Similarly, the piezoelectric effect’s electrostatic charges are thought to contribute in cell-extracellular matrix connections, connective tissue healing during rehabilitation, and mineralization and remodeling of calcified tissues (da Costa Reis and Oliveira 2020; Cerrolaza et al. 2017). Figure 4 describes some biological piezoelectric materials.
Phenomenon such as piezoelectricity arise from the fact that the charge distribution of materials can be asymmetrically induced. At various levels of organization, many, if not all, biological materials possess a spiral/helical form (macromolecules, tissues, etc.). Because spirals/helices lack a center of symmetry, the majority of biological matter, such as wood and bone, has piezoelectric capabilities (Wojnar 2012) in combination with the material’s electromechanical coupling (Jiang et al. 2003). The nervous system, voltage-controlled muscular action, and ion transporters all display piezoelectric properties due to this mechanocoupling linkage (Bystrov et al. 2012). In 1940, Martin measured electric potentials from a bundle of wool crushed by two metal plates, and in doing so he reported the first piezoelectric phenomena in biological tissues (Martin 1941). Keratin, in the shape of an alpha-helix, is the major component of hair, horns, and wool. The highly ordered structure of keratin and the inherent polarization of its component alpha-helices (maintained by hydrogen bonds between the hydrogen in the amine group and the oxygen in the carbonyl group) are known to be responsible for the tissue’s piezoelectricity (Telega and Wojnar2002).
Bone as a piezoelectric material
Bones are made up of two different types of tissue, compact (cortical) bone which constitutes the compact, robust, and hard outer bone layer and accounts for around 80% of adult bone mass(Clarke 2008) and cancellous (spongy or trabecular) bone which exists as a network of trabeculae or rod-like structures often visualized as a spongy meshwork existing at the interior of the bone (Singh 1978). Bones are not static tissues and they must be maintained, remodeled, and reshaped on a regular basis (General 2004). This procedure involves three different cell types:
-
(i)
Osteoblasts: Cells responsible for generating new bones and repair (Tanaka et al. 2005)
-
(ii)
Osteocytes: Inactive osteoblasts important for communication inside bone (Bellido 2014)
-
(iii)
Osteoclasts: Osteoclasts assist in remodeling damaged bones and the creation of channels for nerves and blood vessels to pass through. They produce enzymes and acids to breakdown and digest minerals in bone. This is referred to as resorption (Tanaka et al. 2005).
In 1867, Davis proposed a scientific law stating that soft tissues (e.g., ligaments, tendons, and fascia) heal according to the manner in which they are mechanically stressed (Davis 1867). In 1892, Wolff proposed a similar law in relation to bone transformation (now known as Wolff’s Law) (Wolff 1892) which can be paraphrased as follows:
When healthy human bones are exposed to a shift in mechanical stress over time, they progressively remodel to become stronger and denser to resist the greater loading, or weaker and less dense in response to decreased loading.
In other words, if loads and stress are applied to the bones, the body responds by building up more bone to withstand the force being applied (Frost 1994, Frost 2001). According to the law, there is a strong association between bone density and physical activity. Taken together, Wolf’s and Davis’s laws have created a conundrum for scientists trying to determine how osteocytes and osteoblasts detect and respond to mechanical strain.
In 1957,Yasuda and Fukada reported the presence of a piezoelectric phenomenon in bones (Fukada and Yasuda 1957). In 1960, the role of collagen as piezoelectric polymer in the modeling process was discovered (Ahn and Grodzinsky 2009). Collagen is the most abundant protein in the human body (Halperin et al. 2004). As one of the major building blocks of tissues, it is essential for the normal functioning of the tendon, ligament, bone, cartilage, skin, the heart, and blood vessels. The triple helix is a three-helical spiral form found in collagen molecules. In 1964, Fukada and Yasuda also observed the direct and converse piezoelectric effects in the tendon of horse and ox, which consists of collagen fibers (Fukada and Yasuda 1964). The study attributed the effect to the displacement and polarization of hydrogen bonds present in the polypeptide chains in collagen crystals.
Bone is a composite consisting of about 19 wt% collagen and about 70 wt% hydroxyapatite [HA] and about11 wt% cells, water, and other materials (Kamel et al. 2015b; Zhou and Lee 2011). The major cause of piezoelectricity in living bone has been suggested as being due to the collagen molecule’s non-centrosymmetry (Khare et al. 2020). Collagen fibers experience numerous types of motions during physical activities such as walking, stretching, and climbing, and as a result, bone is subjected to stresses such as compression and tension and in response to such stressors, piezoelectric collagen develops charges (Senior 2010). The direction of mechanical stress or bone deformation determines the polarity of these electrical charges. Negative and positive charges are generated through compression and tension respectively. According to Halperin et al. (2004), the piezoelectric strain coefficient in human tibia varies between 7.7 and 8.7 pC/N. The lack of significant dispersion in piezoelectric strain coefficients suggests that the piezoelectric characteristic is consistent throughout the bone (Magnusson et al. 2001). When collagen molecules are stressed, charge carriers with and surrounding the molecule travel from the interior to the surface generating an electric potential in the bone (Ahn and Grodzinsky 2009, Ferreira et al. 2009). Because of the production of electrical dipoles, this impact attracts bone-building cells known as osteoblasts (Vásquez Sancho 2018). Minerals, principally calcium, are then deposited on the strained side of the bone and as a result of the piezoelectric action, bone density rises (deVet 2020).
Recent research has suggested that bone piezoelectricity is also linked to the piezoelectric characteristics of bone hydroxyapatite. For many years, it was assumed that hydroxyapatite could not be piezoelectric because it crystallizes in a centrosymmetric space group in the hexagonal system. However, computer investigations revealed that hydroxyapatite lacks an inversion center, which would indicate that the crystal is piezoelectric (Rajabi et al. 2015). Other research has indicated that thin films of hydroxyapatite have ferroelectric, piezoelectric, and pyroelectric properties and poled thick films (heat-treated) exhibit strong surface charges with a piezoelectric coefficient as high as that of collagen (Lang 2016).
Piezoelectric biomaterials
Piezoelectric biomaterials are essential in many biomedical applications with most sensor devices associated with the direct effect in contrary, while physical actuators are based on the converse effect (Ali et al. 2019). Piezoelectric materials are used in a wide range of detection-based applications such as detecting specific cancer biomarkers, DNA hybridization detection, determining drug effectiveness, and detection of the hepatitis C virus (Zec et al. 2014; Azhar et al. 2021).
Surface chemistry and topography are well recognized to influence protein adsorption and, as a result, cellular behavior (Anselme et al. 2010; Fadel et al. 2020b). The surface charge and wettability of the scaffolds control interactions at the material-cell interface (Fadel et al. 2020a) (Navarro et al. 2006). The shape of the scaffolds may be controlled using various manufacturing processes, and additional functionalization of the materials can be used to immobilize biochemical substances (Ravichandran et al. 2012). The topographical properties of piezoelectric materials, as well as their piezoelectric constants and ferroelectricity, can be modified to meet particular requirements (Chen et al. 2020a).
Piezoelectric biomaterial applications in tissue regeneration
A range of research has suggested that the electrical potential induced by piezoelectric polymers and ceramics can possibly stimulate the cellular response and bioactivity of hard tissue (Khare et al. 2020). Due to the fact that piezoelectric scaffolds can create electrical impulses in response to mechanical activity on the tissue around the implant, wound healing can be accelerated independently of the implantation zone (Ning et al. 2018).
Piezoelectric phenomena on non-union bone fracture healing
Bone fracture healing is a complicated process and requires the interaction of several factors, including the timely expression of reparative genes and migration and action of certain cells (Bostrom 1998). If these factors are interrupted, the repair process can be impaired and delayed resulting in imperfect (non-union) healing of the bone (Bostrom et al. 1995). Electrical stimulation can be used to aid fracture healing in the majority of instances with this technique being known as capacitive coupling (Abeed et al. 1998). In this method, two skin electrodes are inserted on either side of the broken bone, and a small current is sent between the electrodes by a low voltage battery. The current is not felt by the patient, but it has an impact on the bone cells (Piazzolla et al. 2015).
An ideal bone graft material for bone fractures must exhibit properties conducive to osteointegration, osteoconduction, and osteoinduction (KAMEL et al. 2015a) (Moore et al. 2001). Osteoinduction implies the ability of the material to stimulate stem cell differentiation towards the osteogenic lineage (Polini et al. 2011). Osteoinduction can result from either physical stimulation or the release of bioactive molecules or surface charge production (Lee and Shin 2007). The major benefit of the piezoelectric scaffold is that electrical potential can be created under the effect of mechanical stress without the need of electrodes (Zaszczynska et al. 2020). Karimi et al. (2019) prepared bone scaffold from the piezoelectric polymer poly vinylidene fluoride (PVDF) and nano-hydroxyapatite for naproxen delivery (Karimi et al. 2019). PVDF has a complicated structure and may crystallize into five different crystalline phases: α, β, γ, δ, and ε. α, β, and γ are the most studied phases and in terms of desirable piezoelectric and pyroelectric qualities, the β phase is the most intensively studied (Jia et al. 2017). High amount of piezoelectric β phase in PVDF membranes has been obtained by annealing (Ruan et al. 2018) with studies revealing that the addition of nano-hydroxyapatite improves the scaffold mechanical properties yielding excellent cell viability especially at high extents of piezoelectric β phase.
Zang et al. (2014) fabricated a piezoelectric composite scaffold by freeze casting hydroxyapatite/barium titanate suspensions; the piezoelectric properties were investigated in addition to the morphologies and porosities (Zhang et al. 2014). Results of this study indicated that the porous HA/BT composite possessed a piezoelectric coefficient that was close to, if not higher than, that of native bone. Improvement of the piezoelectric coefficient d33 was effected by increasing the solid loading of the suspension and solidification velocity. According to the cytotoxicity assay on murine fibroblast cells, there were no evidences of cytotoxicity of these composites.
Liu et al. prepared composites from the biocompatible polymer polycaprolactone and barium titanate (BT) particles (Liu et al. 2019) and the effects of changing the BT concentration on the piezoelectric properties of the composite were studied. The composites displayed piezoelectric characteristic due to the presence of BT; in addition, the piezoelectric coefficient (d33) increased with increasing the BT content. This study also confirmed that these types of composite did not display cytotoxicity and were beneficial for cell adhesion.
The cytotoxic and piezoelectric characteristics of a porous lead-free piezoelectric ceramic for usage as a direct bone replacement were studied by Wang et al. (2009). Porous lithium, sodium, potassium, and niobate samples were fabricated by the Cold Isostatic Pressing (CIP) technique (Ani 2006). The ceramic’s influence on the attachment and proliferation of osteoblasts isolated from the skull of 1-day-old Sprague–Dawley rats was investigated and the results confirmed that the CIP preparation technique had positive effects on the attachment and proliferation of osteoblasts and that the niobate ceramic had the potential to be used as a piezoelectric bone scaffold (Wang et al. 2009).
A piezoelectric degradable electrospun poly-l-lactic acid (PLLA) modified polydopamine (PDA) and graphene (rGO) scaffold was developed by Lai et al. (2021) to treat articular cartilage disorder. Results of the study indicated that extracellular matrix secretion can be facilitated by piezoelectricity induced by bending the scaffold with the piezoelectric properties of the scaffold controlling the cell differentiation and proliferation.
Piezoelectric biomaterials in dental applications
Montoya et al. developed a new type of dental resin composite using piezoelectric barium titanate (BaTiO3) nanoparticles as multifunctional bioactive filler in order to offer antibacterial and remineralization effects (Montoya et al. 2021). The smart composite produces electric charges upon activation by the force of mastication (chewing). The produced electric charges were proportional to the concentration of the filler and this was also shown to be proportional to the ability to prevent biofilm formation.
Adaraev et al. synthesized piezoelectric polymer membrane from a vinylidene fluoride/tetrafluoroethylene copolymer coated with a thin layer of copper for use as a wound dressing in surgical procedures involving the oral mucosa (Badaraev et al. 2020). The mechanical, wettability, antibacterial, and other physicochemical properties of these membranes were studied in addition to the in vivo study. Results indicated that this piezoelectric polymer membrane aided in oral mucosa regeneration and this process additionally acted as an antibacterial coating.
Piezoelectric biomaterials for cochlea implantation
The mammalian cochlea, which is a structure of the inner ear (Fig. 5), is an organ that converts incoming mechanical energy into electrical impulses in the auditory nerve fibers that terminate within it and, in actuality, it is the most sensitive and advanced acoustic sensor ever devised (Mukherjee et al. 2000). Sensorineural hearing loss (SNHL) originates from structural damage occurring to the auditory nerve and SNHL is the cause of more than 90% of deafness problems (Hopkins2015). The origin of SNHL may be due to genetic factors, exposure to loud noises, or aging (Hannaford et al. 2005). Cochlear implants are a type of hearing aid that has been created to help people with sensorineural hearing loss. The process of artificial electrical stimulation of auditory nerve fibers within the cochlea can be substantially simplified using a piezoelectric cochlear implant and research has been conducted to investigate their use in place of hair cells to create an artificial cochlear epithelium (Inaoka et al. 2011).
Structure of the ear (the outer, middle, and inner ear) (produced with permission from McKnight 2012)
Jung et al. constructed a piezoelectric artificial cochlea (PAC) that is capable of interpreting incoming vibratory signals across the human hearing range without the need for additional power (Jung et al. 2015) with the human cochlea influencing the design, components, and operation of the PAC. The PAC was composed of a corona-poled piezoelectric thin film with a unique vibrating membrane. The frequency separation of the produced PAC was determined by measuring the vibration displacement of the membrane using a laser Doppler vibrometer to analyze the results. Mechanical vibratory behavior experiments showed that incoming signals could be separated into 13 different frequency bands based on their frequency range of 300 to 6000 Hz.
Piezoelectric biomaterials for nerve regeneration
Neurotrauma is a leading risk factor for mortality around the world and is a major public health problem (Rubiano et al. 2015) (Reilly 2007). A variety of biomaterials have shown promise in enhancing and/or repairing the function of damaged nervous system tissue (Chen et al. 2019). However, considerable obstacles remain in the way of full functional recovery of nervous system tissue, such as those concerned with delayed or partial tissue regeneration (Khaing et al. 2014). More recent approaches to designing the next generation of tissue engineering scaffolds for the nervous system have incorporated nanotechnology or, more specifically, nanoscale surface feature dimensions that mimic natural neural tissue, and to this point piezoelectric scaffolds, considered smart scaffolds, have shown great potential in the noninvasive treatment of neural tissue (Zaszczynska et al. 2020).
Seil and Webster studied the activity of astroglial cells on polyurethane-ZnO composites and the astroglial cell response was evaluated depending on cell adhesion and proliferation (Seil and Webster, 2008). Astrocyte adhesion was significantly reduced on ZnO NPs/[PU] composites compared to pure PU indicating that ZnO-PU composite scaffolds have the potential to facilitate nerve regeneration with greater efficiency than what is available today (Fig. 6).
Schematic representation of nerve regeneration. a Peripheral nerve injury. b Wallerian degeneration, fragmentation of the axon, and myelin sheath. Schwann cells proliferate and phagocytosis of the degrading materials by macrophages. c Schwann cells in the distal segment line up in bands of Büngner. d Axonal reconnected and nerve fiber remyelinated (
A piezoelectric nanogenerating scaffold of zinc oxide was fabricated by Qian et al. (2020). The piezoelectric scaffold was shown to exhibit suitable physical and mechanical characteristics as well as biocompatibility. Results of the study suggested that the piezoelectric conduit accelerated the nerve conduction velocity and stimulated axonal remyelination, restoring motor function through promoting the recovery of end plate muscles. Furthermore, the piezoelectric nanogenerator scaffold created a microenvironment that is electrically conductive and biomimetic without causing any toxicity to functional organs.
Piezoelectric biosensors
A biosensor is an analytical device that measures chemical or biological reactions by producing signals proportionate to the concentration of the analyte in the reaction (Turner 2013). Biosensors can be divided into various groups, such as electrochemical, optical, and piezoelectric (Marco and Barceló 1996). In general, there are three components of a biosensor (Fig. 7) (Pohanka and Skládal 2008):
-
(i)
Analyte—the investigation biological molecule that must be identified by the device
-
(ii)
Receptor—a biomolecule that recognizes a target molecule (the analyte)
-
(iii)
Transducer—a device for converting the recognition event into a measurable signal.
Biosensors are employed in applications such as drug discovery, disease monitoring, monitoring of disease-causing micro-organisms, and the detection of pollutants and markers which act as indicators of a disease in body fluids (e.g., sweat, blood, saliva, urine) (Schmid and Scheller 1989). As discussed within this review, piezoelectricity is highly suited to the fabrication of physical sensors and biosensors. It can easily record affinity interactions without requiring the use of any special reagents; however, the required loading sensitivity can often be in the range micrograms, due to the requirement to generate a noticeable change in oscillation frequency of the piezoelectric device (Pohanka 2018).
Piezoelectric biosensors for breast cancer detection
Investigation of early stages of breast cancer has been carried out using many diagnostic tools such as magnetic resonance imaging, positron emission tomography, mammography, ultrasound, computerized tomography, and biopsy (Wang 2017a). Even so, these techniques still have some limitations like being expensive and time-consuming (Pereira et al. 2019) and therefore, it is critical to develop a high-sensitivity and quick detection technique.
Sensitive piezoelectric sensors constructed using receptor antibodies that specifically bind to human epidermal growth factor receptor (HER2) have been created by Loo et al. for use in breast cancer detection (Loo et al. 2011). A piezoelectric microcantilever sensor (PEM) was developed to detect HER2 biomarker levels present in human blood samples of patients with breast cancer. The anti-HER2 PEM biosensor was able to detect the naturally occurring and recombinant HER2 accurately at clinically relevant levels (> 2 ng/mL) affirming that the PEM biosensor is an effective tool for breast cancer detection.
A household usable device for early diagnosis and self-screening of breast cancer, based on quartz crystal microbalance biosensor, was introduced by Arif et al. (2015). The sensor worked using a saliva sample which represents a noninvasive routine to detect the ATP6AP1 auto-antibodies that appear during the early stages of breast cancer and as such the device may serve as alert to women instead of the regularized check-ups.
A PZT ceramic-based biosensor was developed by Ramirez et al. for the detection of cancer markers (Ramirez-Valles et al. 2020). The detection of the cancer biomarkers α-fetoprotein (AFP) and prostate-specific antigen (PSA) was carried out through monitoring a change in frequency. The biosensor showed high-sensitivity and fast detection with small sample. In addition, the results indicated that the ceramic piezoelectric biosensor could be used with various chemical interfaces with the possibility that the biosensor array could be expanded to different specificities for simultaneous detection of a range of analytes.
A piezoelectric finger (PEF) is a radiation-free, portable, and low-cost breast tumor detector capable of electrical tissue stiffness measurement (Xu et al. 2013). Measurement of tissue stiffness is achieved by simply placing a PEF on a tissue and inducing palpation type t (electronic palpation) but with much higher sensitivity and accuracy than is possible via other approaches (Xu et al. 2016). PEF has been successfully tested in vivo with better sensitivity than mammography and has been shown to be able to detect breast cancers in women with dense breasts that were undetectable via mammography (Xu et al. 2016). The UELS has licensed the technology and received FDA approval with the PEF breast cancer detector marketed under the trade name intelligent Breast Exam (iBE®) (Mango et al. 2022).
Piezoelectric biosensors for detection of viral infection
Under an alternating current (AC), a piezoelectric material shows mechanical oscillation. The frequency regulated by the AC voltage drops as the mass m grows owing to molecular interactions. For viral detection, mass response-type piezoelectric sensors are often utilized (Chen et al. 2020b). The global spread of the severe acute respiratory syndrome coronavirus has reinforced the need for fast and sensitive detection techniques for controlling the outbreak (Aloraini et al. 2021). The techniques (and their limitations) for virus detection using different virus sensors have been reviewed by Narita et al. (2021).
Kabir et al. developed a piezoelectric microcantilever antibody-based biosensor for detecting COVID-19 without the need for any prior processing (Kabir et al. 2021). When the SARS-CoV-2 spike protein antigens adsorb on the top surface of the microcantilever, a surface tension is induced owing to the mass shift, resulting in observable tip deflection. Different forms and piezoelectric materials were evaluated to create a biosensor with optimal characteristics, and it was determined that a poly[vinylidene fluoride] (PVDF) biosensor in the shape of a holed punched form triangle represented the best outcome. As a result, COVID-19 may be detected quickly in clinical samples with varying virus loads using the extremely sensitive microcantilever biosensor.
Wang et al. also studied the dynamic electromechanical response of piezoelectric fiber-epoxy matrix composites as mass load sensors for COVID-19 detection (Wang et al. 2021). Although the preceding literature indicates great potential, the construction of a biosensor with optimum parameters for the detection of a specific virus with dependable accuracy remains a challenge for the future.
Conclusions
This review has summarized the piezoelectric effect phenomena exploring the piezoelectric properties of both non-biological and biological materials with particular attention to piezoelectric properties of bones and history and types of piezoelectric biomaterials and highlights their applications in tissue engineering and biosensors. Piezoelectrics are non-centrosymmetric materials that can behave like a mechano-electrical transducer. Piezoelectric materials represent the second major application of dielectric materials after semiconductors. They have many applications in the biomedical field and hold great potential as osteoinductive scaffolds in tissue regeneration. Electroactive scaffolds can create an electric field as a response of mechanical vibrations. They can also be tuned to create electric field properties of the original extracellular matrix to modulate the signaling pathways. Such approaches offer the potential for extending traditional techniques for inducing differentiation and proliferation of cells, such as through the incorporation of drugs or stimulating factors, which can be expensive and complicated.
In addition, piezoelectricity concepts are used widely in the manufacture of several devices such as sensors and actuators. The accuracy of detection and sensitivity by piezoelectric sensors is comparable with optical and electrochemical detectors. Apart from these real-time and label-free qualities, the fundamental advantage of piezoelectric biosensors is their flexibility. In comparison to other sensors, such as optical devices, this method is flexible and inexpensive. As seen in this review, piezoelectric-based biosensors can even be used in the detection of viral pathogens such as the virus responsible for COVID-19.
In summary, piezoelectric materials will be important for future progress in biomedical science and regenerative medicine, and therefore, attention must be paid to the future development of this group of self-controlling smart systems.
References
Aaron RK, Ciombor DM, Simon BJ (2004) Treatment of nonunions with electric and electromagnetic fields. Clin Orthop Relat Res 1976–2007(419):21–29. https://doi.org/10.1097/00003086-200402000-00005
Abeed R, Naseer M, Abel E (1998) Capacitively coupled electrical stimulation treatment: results from patients with failed long bone fracture unions. J Orthop Trauma 12:510–513. https://doi.org/10.1097/00005131-199809000-00015
Ahamed R, Choi S-B, Ferdaus MM (2018) A state of art on magneto-rheological materials and their potential applications. J Intell Mater Syst Struct 29:2051–2095. https://doi.org/10.1177/1045389X18754350
Ahn AC, Grodzinsky Aj (2009) Relevance of collagen piezoelectricity to “Wolff’s Law”: a critical review. Med Eng Phys 31:733–741. https://doi.org/10.1016/j.medengphy.2009.02.006
Akdogan EK, Allahverdi M, Safari A (2005) Piezoelectric composites for sensor and actuator applications. IEEE Trans Ultrason Ferroelectr Freq Control 52:746–775. https://doi.org/10.1109/TUFFC.2005.1503962
Akhras G (2000) Smart materials and smart systems for the future. Canadian Military J 1:25–31
Ali F, Raza W, Li X, Gul HK, K-H. (2019) Piezoelectric energy harvesters for biomedical applications. Nano Energy 57:879–902. https://doi.org/10.1016/j.nanoen.2019.01.012
Aloraini DA, Almuqrin AH, Alanazi A, Ain QT, Alodhayb AN (2021) Rapid and sensitive detection of severe acute respiratory syndrome coronavirus 2 in label-free manner using micromechanical sensors. Sensors 21:4439. https://doi.org/10.3390/s21134439
Ani, SM. 2006. Physical behaviour of powder ceramic part using Cold Isostatic Pressing (CIP) processes. Universiti Teknologi Malaysia.
Anju, M., Raj, DK., Madathil, BK., Kasoju, N. Anil Kumar, P. 2021. Intelligent biomaterials for tissue engineering and biomedical applications: current landscape and future prospects. Biomater Tissue Eng Regenerative Med. Springer. https://doi.org/10.1007/978-981-16-0002-9_16
Anselme K, Ploux L, Ponche A (2010) Cell/material interfaces: influence of surface chemistry and surface topography on cell adhesion. J Adhes Sci Technol 24:831–852. https://doi.org/10.1163/016942409X12598231568186
Arif S, Qudsia S, Urooj S, Chaudry N, Arshad A, Andleeb S (2015) Blueprint of quartz crystal microbalance biosensor for early detection of breast cancer through salivary autoantibodies against ATP6AP1. Biosens Bioelectron 65:62–70. https://doi.org/10.1016/j.bios.2014.09.088
Arnau A, Soares D (2009) Fundamentals of piezoelectricity. Springer, Piezoelectric transducers and applications
Azhar A, Hassan N, Singh M, Al-Hossaini K, Kamal MA (2021) Synopsis of pharmotechnological approaches in diagnostic and management strategies for fighting against COVID-19. Curr Pharm Des 27:4086–4099. https://doi.org/10.2174/1381612827666210715154004
Badaraev AD, Koniaeva A, Krikova SA, Shesterikov EV, Bolbasov EN, Nemoykina AL et al (2020) Piezoelectric polymer membranes with thin antibacterial coating for the regeneration of oral mucosa. Appl Surf Sci 504. https://doi.org/10.1016/j.apsusc.2019.144068
Bell AJ, Deubzer O (2018) Lead-free piezoelectrics—the environmental and regulatory issues. MRS Bull 43:581–587. https://doi.org/10.1557/mrs.2018.154
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94:25–34https://doi.org/10.1007/s00223-013-9774-y
Binyamin G, Shafi BM, Mery CM (2006) Biomaterials: a primer for surgeons. Seminars in pediatric surgery, Elsevier, 276–283. https://doi.org/10.1053/j.sempedsurg.2006.07.007
Bostrom MP (1998) Expression of bone morphogenetic proteins in fracture healing. Clin Orthopaedics Related Res®, 355, S116-S123. https://doi.org/10.1097/00003086-199810001-00013
Bostrom MP, Lane JM, Berberian WS, Missri AA, Tomin E, Weiland A, Doty SB, Glaser D, Rosen VM (1995) Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res 13:357–367. https://doi.org/10.1002/jor.1100130309
Brankovic Z, Brankovic G, Jovalekic Č, Maniette Y, Cilense M, Varela JA (2003) Mechanochemical synthesis of PZT powders. Materials Science and Engineering: A, 345, 243–248. https://doi.org/10.1002/jor.1100130309
Broadhurst M, Davis G, Mckinney J, Collins R (1978) Piezoelectricity and pyroelectricity in polyvinylidene fluoride—a model. J Appl Phys 49:4992–4997
Bystrov VS, Bdikin IK, Heredia A, Pullar RC, Mishina ED, Sigov AS, Kholkin AL (2012) Piezoelectricity and ferroelectricity in biomaterials: from proteins to self-assembled peptide nanotubes. In: Ciofani G, Menciassi A (eds) Piezoelectric nanomaterials for biomedical applications. Nanomedicine and Nanotoxicology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28044-3_7
Cerrolaza M, Duarte V, Garzon-Alvarado D (2017) Analysis of bone remodeling under piezoelectricity effects using boundary elements. J Bionic Eng 14:659–671. https://doi.org/10.1016/S1672-6529(16)60432-8
Chaudhari R, Vora JJ, Parikh DM (2021) A Review on applications of nitinol shape memory alloy. In: Parwani AK, Ramkumar P, Abhishek K, Yadav SK (eds) Recent advances in mechanical infrastructure. Lecture Notes in Intelligent Transportation and Infrastructure. Springer, Singapore. https://doi.org/10.1007/978-981-33-4176-0_10
Chen C, Wang X, Wang Y, Yang D, Yao F, Zhang W, Wang B, Sewvandi GA, Yang D, Hu D (2020) Additive manufacturing of piezoelectric materials. Adv Func Mater 30:2005141. https://doi.org/10.1002/adfm.202005141
Chen S, Auriat AM, Li T, Stumpf TR, Wylie R, Chen X, Willerth SM, Derosa M, Tarizian M, Cao X (2019) Advancements in Canadian biomaterials research in neurotraumatic diagnosis and therapies. Processes 7:336. https://doi.org/10.3390/pr7060336
Chen Y, Qian C, Liu C, Shen H, Wang Z, Ping J, Wu J, Chen H (2020) Nucleic acid amplification free biosensors for pathogen detection. Biosens Bioelectron 153:112049. https://doi.org/10.1016/j.bios.2020.112049
Ciofani G, Danti S, Ricotti L, D’allessandro D, Moscato S, Berrettini S, Mattoli V, Menciassi A (2011) Boron nitride nanotubes: production, properties, biological interactions and potential applications as therapeutic agents in brain diseases. Curr Nanosci 7:94–109. https://doi.org/10.2174/157341311794480345
Ciofani, G. Menciassi, A. 2012. Piezoelectric nanomaterials for biomedical applications. Springer Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-28044-3
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3:S131–S139. https://doi.org/10.2215/CJN.04151206
Cordero Edwards K, Domingo N, Abdollahi A, Sort J, Catalan G (2017) Ferroelectrics as smart mechanical materials. Adv Mater 29:1702210. https://doi.org/10.1002/adma.201702210
Covaci C, Gontean A (2020) Piezoelectric energy harvesting solutions: a review. Sensors 20:3512. https://doi.org/10.3390/s20123512
Cross L, Newnham R (1987) Hist Ferroelectrics Ceramics Civilization 3:289–305
Curry EJ, Ke K, Chorsi MT, Wrobel KS, Miller AN, Patel A, Kim I, Feng J, Yue L, Wu Q (2018) Biodegradable piezoelectric force sensor. Proc Natl Acad Sci 115:909–914
Da Costa Reis J, Oliveira MT (2020) Bone: functions, structure and physiology. In: Belinha J, Manzanares-Céspedes MC, Completo A (eds) The computational mechanics of bone tissue. Lecture Notes in Computational Vision and Biomechanics, vol 35. Springer, Cham. https://doi.org/10.1007/978-3-030-37541-6_1
Davis HG (1867) Conservative surgery. Appleton
Devet, T. 2020. Electrical stimulation in bone cell culture media.
Dineva P, Gross D, Muller R, Rangelov T (2014) Piezoelectric materials. Springer, Dynamic fracture of piezoelectric materials
Dong K, Peng X, Wang ZL (2020) Fiber/fabric-based piezoelectric and triboelectric nanogenerators for flexible/stretchable and wearable electronics and artificial intelligence. Adv Mater 32:1902549. https://doi.org/10.1002/adma.201902549
Duerig, T., Stoeckel, D. Johnson, D. 2003 SMA: smart materials for medical applications. European Workshop on Smart Structures in Engineering and Technology. International Soc Optics and Photonics, 7–15.
Ebara, M., Kotsuchibashi, Y., Narain, R., Idota, N., Kim, Y-J., Hoffman, JM., Uto, K. Aoyagi, T. 2014. Smart biomaterials. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54400-5
Enderle, JD. 2005. Introduction to biomedical engineering second edition.
Erhart J, Privratska J (2010) Principles of piezoelectricity. Springer, Fundamentals of Piezoelectric Sensorics
Fadel MA, Kamel NA, Darwish MM, El-Messieh A, Salwa L, Abd-el-Nour KN, Khalil WA (2020a) Effect of microwave treatment on biophysical and surface properties of polyethylene terephthalate (PET) for blood contact applications. Proceed National Academy Sci, India Section B: Biol Sci 90:343–351. https://doi.org/10.1007/s40011-019-01107-8
Fadel MA, Kamel NA, Darwish MM, El-Messieh SLA, Abd-el-Nour KN, Khalil WA (2020b) Preparation and characterization of polyethylene terephthalate–chamomile oil blends with enhanced hydrophilicity and anticoagulant properties. Prog Biomater 9:97–106. https://doi.org/10.1007/s40204-020-00133-4
Fairman RÅ, Åkerfeldt KS (2005) Peptides as novel smart materials. Curr Opin Struct Biol 15:453–463. https://doi.org/10.1016/j.sbi.2005.07.005
MY., Mitchell, GR., Alves, N. Morouco, P. 2019 Smart materials for biomedical applications: the usefulness of shape-memory polymers. Applied Mechanics and Materials, Trans Tech Publ, 237–247.
Ferreira, A., Gonzalez, G., Gonzalez -Paz, R., Feijoo, J., Lira-Olivares, J. Noris-Suarez, K. 2009. Bone collagen role in piezoelectric mediated remineralization. Acta Microsc 18:278–286
Finazzi G, Petroutsos D, Tomizioli M, Flori S, Sautron E, Villanova V, Rolland N, Seigneurin-Berny D (2015) Ions channels/transporters and chloroplast regulation. Cell Calcium 58:86–97. https://doi.org/10.1016/j.ceca.2014.10.002
Frost HM (1994) Wolff’s Law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod 64:175–188
Fost HM (2001) From Wolff’s law to the Utah paradigm: insights about bone physiology and its clinical applications. Anatomical Record: Official Publ American Assoc Anatomists 262:398–419. https://doi.org/10.1002/ar.1049
Fukada E (1995) Piezoelectricity of biopolymers. Biorheology 32:593–609
Fukada E (2000) History and recent progress in piezoelectric polymers. IEEE Trans Ultrason Ferroelectr Freq Control 47:1277–1290. https://doi.org/10.1109/58.883516
Fukada E, Yasuda I (1957) On the piezoelectric effect of bone. J Phys Soc Jpn 12:1158–1162
Fukada E, Yasuda I (1964) Piezoelectric effects in collagen. Jpn J Appl Phys 3:117
Gao S, Tang G, Hua D, Xiong R, Han J, Jiang S, Zhang Q, Huang C (2019) Stimuli-responsive bio-based polymeric systems and their applications. J Mater Chem B 7:709–729. https://doi.org/10.1039/C8TB02491J
Garcia Huete, N. 2017. Development and applications of semicrystalline polymers: from shape memory to self-healing materials.
Gautschi G (2002) Background of piezoelectric sensors. In: Piezoelectric Sensorics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-04732-3_2
General, OOTS. 2004. The basics of bone in health and disease. Bone Health and Osteoporosis: A Report of the Surgeon General. Office of the Surgeon General (US).
Genet S, Costalat R, Burger J (2000) A few comments on electrostatic interactions in cell physiology. Acta Biotheor 48:273–287. https://doi.org/10.1023/A:1010229531210
Goel P, Yadav K (2005) A comparative analysis of PBZT synthesized by co-precipitation and sol-gel method
Gonzalez M (2016) Impact of Li non-stoichiometry on the performance of acoustic devices on LiTaO3 and LiNbO3 single crystals. Université de Franche-Comté
Gross S, Tadigadapa S, Jackson T, Trolier-Mckinstry S, Zhang Q (2003) Lead-zirconate-titanate-based piezoelectric micromachined switch. Appl Phys Lett 83:174–176. https://doi.org/10.1063/1.1589192
Guinier A (1994) X-ray diffraction in crystals, imperfect crystals, and amorphous bodies, Courier Corporation
Guo Z, Liu H, Dai W, Lei Y (2020) Responsive principles and applications of smart materials in biosensing. Smart Mater Med 1:54–65. https://doi.org/10.1016/j.smaim.2020.07.001
Halperin C, Mutchnik S, Agronin A, Molotskii M, Urenski P, Salai M, Rosenman G (2004) Piezoelectric effect in human bones studied in nanometer scale. Nano Lett 4:1253–1256. https://doi.org/10.1021/nl049453i
Hannaford PC, Simpson JA, Bisset AF, Davis A, Mckerrow W, Mills R (2005) The prevalence of ear, nose and throat problems in the community: results from a national cross-sectional postal survey in Scotland. Fam Pract 22:227–233. https://doi.org/10.1093/fampra/cmi004
Hocker H, Klee D (1996) Polymers for medical uses. Macromolecular Symposia, Wiley Online Library 421–427. https://doi.org/10.1002/masy.19961020149
Honig B, Nicholls A (1995) Classical electrostatics in biology and chemistry. Science 268:1144–1149. https://doi.org/10.1126/science.7761829
Hopkins K (2015) Deafness in cochlear and auditory nerve disorders. Elsevier, Handbook of Clinical Neurology. https://doi.org/10.1016/B978-0-444-62630-1.00027-5
Hu Y, Kang W, Fang Y, Xie L, Qiu L, Jin T (2018) Piezoelectric poly (vinylidene fluoride)(PVDF) polymer-based sensor for wrist motion signal detection. Appl Sci 8:836. https://doi.org/10.3390/app8050836
Inaoka T, Shintaku H, Nakagawa T, Kawano S, Ogita H, Sakamoto T, Hamanishi S, Wada H, Ito J (2011) Piezoelectric materials mimic the function of the cochlear sensory epithelium. Proc Natl Acad Sci 108:18390–18395. https://doi.org/10.1073/pnas.1110036108
Iwazumi T, Noble M (1989) An electrostatic mechanism of muscular contraction. Int J Cardiol 24:267–275. https://doi.org/10.1016/0167-5273(89)90003-X
Jacob J, More N, Kalia K, Kapusetti G (2018) Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflam Regen 38:1–11. https://doi.org/10.1186/s41232-018-0059-8
Jacob KS, Panicker NR, Selvam IP, Kumar V (2003) Sol-gel synthesis of nanocrystalline PZT using a novel system. J Sol-Gel Sci Technol 28:289–295. https://doi.org/10.1023/A:1027457929302
Jia N, He Q, Sun J, Xia G, Song R (2017) Crystallization behavior and electroactive properties of PVDF, P (VDF-TrFE) and their blend films. Polym Testing 57:302–306. https://doi.org/10.1016/j.polymertesting.2016.12.003
Jiang W, Zhang R, Jiang B, Cao W (2003) Characterization of piezoelectric materials with large piezoelectric and electromechanical coupling coefficients. Ultrasonics 41:55–63. https://doi.org/10.1016/S0041-624X(02)00436-5
Jung Y, Kwak J-H, Kang H, Kim W, Hur S (2015) Development of piezoelectric artificial cochlea inspired by human hearing organ. Conference on Biomimetic and Biohybrid Systems, Springer 145–152. https://doi.org/10.1007/978-3-319-22979-9_15
Kabir H, Merati M, Abdekhodaie MJ (2021) Design of an effective piezoelectric microcantilever biosensor for rapid detection of COVID-19. J Med Eng Technol 1–11. https://doi.org/10.1080/03091902.2021.1921067
Kamel NA, Abd-el-Messieh SL, Mansour SH, Abd-el-Nour K (2015a) Effect of incorporation of bone powder on the physical properties of polypropylene fumarate based bone cement. Rom J Biophys 25
Kamel NA, Mansour SH, Abd-el-Messieh SL, Khalil WA, Abd-el-Nour KN (2015b) Biophysical properties of PPF/HA nanocomposites reinforced with natural bone powder. Adv Mater Res 4:145. https://doi.org/10.12989/amr.2015.4.3.145
Kapat K, Shubhra QT, Zhou M, Leeuwenburgh S (2020) Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv Func Mater 30:1909045. https://doi.org/10.1002/adfm.201909045
Karimi S, Ghaee A, Barzin J (2019) Preparation and characterization of a piezoelectric poly (vinylidene fluoride)/nanohydroxyapatite scaffold capable of naproxen delivery. Eur Polymer J 112:442–451. https://doi.org/10.1016/j.eurpolymj.2019.01.027
Khaing ZZ, Thomas RC, Geissler SA, Schmidt CE (2014) Advanced biomaterials for repairing the nervous system: what can hydrogels do for the brain? Mater Today 17:332–340. https://doi.org/10.1016/j.mattod.2014.05.011
Khare D, Basu B, Dubey AK (2020) Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials 258:120280. https://doi.org/10.1016/j.biomaterials.2020.120280
Kholkin AL, Pertsev NAG, AV. (2008) Piezoelectricity and crystal symmetry. Springer, Piezoelectric and Acoustic Materials for Transducer Applications. https://doi.org/10.1007/978-0-387-76540-2_2
Kim K (2013) Novel shear mode piezoelectric sensors and their applications
Koptsik VA, Rez I (1981) Pierre Curie’s works in the field of crystal physics (on the one-hundredth anniversary of the discovery of the piezoelectric effect). Soviet Phys Uspekhi 24:426. https://doi.org/10.1070/PU1981v024n05ABEH004861
Lai Y-H, Chen Y-H, Pal A, Chou S-H, Chang S-J, Huang E-W, Lin Z-H, Chen S-Y (2021) Regulation of cell differentiation via synergistic self-powered stimulation and degradation behavior of a biodegradable composite piezoelectric scaffold for cartilage tissue. Nano Energy 90:106545. https://doi.org/10.1016/j.nanoen.2021.106545
Lang SB (2016) Review of ferroelectric hydroxyapatite and its application to biomedicine. Phase Transitions 89:678–694. https://doi.org/10.1080/01411594.2016.1182166
Lay R, Deijs GSM, J. (2021) The intrinsic piezoelectric properties of materials–a review with a focus on biological materials. RSC Adv 11:30657–30673. https://doi.org/10.1039/D1RA03557F
Lee JC, Suh IW, Park CH, Kim CS (2021) Polyvinylidene fluoride/silk fibroin-based bio-piezoelectric nanofibrous scaffolds for biomedical application. J Tissue Eng Regen Med 15:869–877. https://doi.org/10.1002/term.3232
Lee S-H, Shin H (2007) Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59:339–359. https://doi.org/10.1016/j.addr.2007.03.016
Leung C, Kinns H, Hoogenboom BW, Howorka S, Mesquida P (2009) Imaging surface charges of individual biomolecules. Nano Lett 9:2769–2773. https://doi.org/10.1021/nl9012979
Li J-F (2020) Lead-Free Piezoelectric Materials. John Wiley & Sons
Liu J, Gu H, Liu Q, Ren L, Li G (2019) An intelligent material for tissue reconstruction: the piezoelectric property of polycaprolactone/barium titanate composites. Mater Lett 236:686–689. https://doi.org/10.1016/j.matlet.2018.11.036
Loo L, Capobianco JA, Wu W, Gao X, Shih WY, Shih W-H, Pourrezaei K, Robinson MK, Adams GP (2011) Highly sensitive detection of HER2 extracellular domain in the serum of breast cancer patients by piezoelectric microcantilevers. Anal Chem 83:3392–3397. https://doi.org/10.1021/ac103301r
Magnusson SP, Aagaard P, Rosager S, Dyhre-Poulsen P, Kjaer M (2001) Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 531:277–288. https://doi.org/10.1111/j.1469-7793.2001.0277j.x
Main JA, Garcia E, Newton DV (1995) Precision position control of piezoelectric actuators using charge feedback. J Guid Control Dyn 18:1068–1073. https://doi.org/10.2514/3.21506
Maiti S, Karan SK, Kim JKK, BB. (2019) Nature driven bio-piezoelectric/triboelectric nanogenerator as next-generation green energy harvester for smart and pollution free society. Adv Energy Mater 9:1803027. https://doi.org/10.1002/aenm.201803027
Makhatadze GI (2017) Linking computation and experiments to study the role of charge–charge interactions in protein folding and stability. Phys Biol 14:013002. https://doi.org/10.1088/1478-3975/14/1/013002
Mango VL, Olasehinde O, Omisore AD, Wuraola FO, Famurewa OC, Sevilimedu V, Knapp GC, Steinberg E, Akinmaye PR, Adewoyin BD (2022) The iBreastExam versus clinical breast examination for breast evaluation in high risk and symptomatic Nigerian women: a prospective study. Lancet Glob Health 10:e555–e563. https://doi.org/10.1016/S2214-109X(22)00030-4
Marco M-P, D. (1996) Environmental applications of analytical biosensors. Meas Sci Technol 7:1547. https://doi.org/10.1088/0957-0233/7/11/002
Marino A, Gross B (1989) Piezoelectricity in cementum, dentine and bone. Arch Oral Biol 34:507–509. https://doi.org/10.1016/0003-9969(89)90087-3
Marshall JM, Dimova-Malinovska D (2002) Photovoltaic and photoactive materials: properties, technology and applications. Springer Science & Business Media. https://doi.org/10.1007/978-94-010-0632-3
Martin A (1941) Tribo-electricity in wool and hair. Proceed Phys Soc (1926–1948) 53:186. https://doi.org/10.1088/0959-5309/53/2/310
Mason W (1946) The elastic, piezoelectric, and dielectric constants of potassium dihydrogen phosphate and ammonium dihydrogen phosphate. Phys Rev 69:173. https://doi.org/10.1103/PhysRev.69.173
Mccabe JF, Yan Z, Al Naimi O, Mmahmoud G, Rolland S (2011) Smart materials in dentistry. Aust Dent J 56:3–10. https://doi.org/10.1111/j.1834-7819.2010.01291.x
Mcknight CL (2012) Vibratory response of dry human skulls
Miao H, Li F (2015) Realization of face-shear piezoelectric coefficient d36 in PZT ceramics via ferroelastic domain engineering. Appl Phys Lett 107:122902. https://doi.org/10.1063/1.4931685
Montoya C, Jain A, Londoὴ JJ, Correa S, Lelkes PI, Melo MA, Orrego S (2021) Multifunctional dental composite with piezoelectric nanofillers for combined antibacterial and mineralization effects. ACS Appl Mater Interfaces 13:43868–43879. https://doi.org/10.1021/acsami.1c06331
Moore WR, Graves SE, Bain GI (2001) Synthetic bone graft substitutes. ANZ J Surg 71:354–361. https://doi.org/10.1046/j.1440-1622.2001.02128.x
Mukherjee N, Roseman RD, Willging JP (2000) The piezoelectric cochlear implant: concept, feasibility, challenges, and issues. J Biomed Mater Res: Official J Soc Biomater, Japanese Soc Biomater, Australian Soc Biomater Korean Soc Biomater 53:181–187. https://doi.org/10.1002/(SICI)1097-4636(2000)53:2<181::AID-JBM8>3.0.CO;2-T
Murayama N, Obara H (1983) Piezoelectric polymers and their applications. Jpn J Appl Phys 22:3. https://doi.org/10.7567/JJAPS.22S3.3
Nandhini A, Sudhakar T, Premkumar J (2021) Ceramics and nanoceramics in biomedical applications. Springer, Handbook of Polymer and Ceramic Nanotechnology. https://doi.org/10.1007/978-3-030-40513-7_71
Narita F, Wang Z, Kurita H, Li Z, Shi Y, Jia Y, Soutis C (2021) A review of piezoelectric and magnetostrictive biosensor materials for detection of COVID-19 and other viruses. Adv Mater 33:2005448. https://doi.org/10.1002/adma.202005448
Navarro M, Aparicio C, Charles-Harris M, Ginebra M, Engel E, Planell J (2006) Development of a biodegradable composite scaffold for bone tissue engineering: physicochemical, topographical, mechanical, degradation, and biological properties. Ordered Polymeric Nanostructures at Surfaces 209–231. https://doi.org/10.1007/12_068
Nerkar PS, Tawale SJ, Saoji SM. Doye AD (2022) Evaluation of smart bio-materials in orthopedics and tissue engineering. Proceedings of the International Conference on Industrial and Manufacturing Systems (CIMS-2020). Springer, p 587–600. https://doi.org/10.1007/978-3-030-73495-4_40
Ning C, Zhou Z, Tan G, Zhu Y, Mao C (2018) Electroactive polymers for tissue regeneration: developments and perspectives. Prog Polym Sci 81:144–162. https://doi.org/10.1016/j.progpolymsci.2018.01.001
Nour E, Nur O, Willander M (2017) Zinc oxide piezoelectric nanogenerators for low frequency applications. Semicond Sci Technol 32:064005. https://doi.org/10.1088/1361-6641/aa6bde
Otsuka K, Wayman CM (1999) Shape memory materials, Cambridge university press
Panda P, Sahoo B (2015) PZT to lead free piezo ceramics: a review. Ferroelectrics 474:128–143. http://www.worldcat.org/oclc/301078063
Park IW, Kim KW, Hong Y, Yoon HJ, Lee Y, Gwak D, Heo K (2020) Recent developments and prospects of M13-bacteriophage based piezoelectric energy harvesting devices. Nanomaterials 10:93. https://doi.org/10.3390/nano10010093
Pereira A, Sales M, Rodrigues L (2019) Biosensors for rapid detection of breast cancer biomarkers. Elsevier, Advanced biosensors for health care applications. https://doi.org/10.1016/B978-0-12-815743-5.00003-2
Petrini L, Migliavacca F (2011) Biomedical applications of shape memory alloys. J Metallurgy 2011. https://doi.org/10.1155/2011/501483
Piazzolla A, Solarino G, Bizzoca D, Garofalo N, Dicuonzo F, Setti S, Moretti B (2015) Capacitive coupling electric fields in the treatment of vertebral compression fractures. J Biol Regul Homeost Agents 29:637–646
Pohanka M (2018) Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 11:448. https://doi.org/10.3390/ma11030448
Pohanka, M. Skladal, P (2008) Electrochemical biosensors--principles and applications. J Appl Biomed 6. https://doi.org/10.32725/jab.2008.008
Poillot P, Le Maitre CL, Huyghe JM (2021) The strain-generated electrical potential in cartilaginous tissues: a role for piezoelectricity. Biophys Rev 13:91–100. https://doi.org/10.1007/s12551-021-00779-9
Polini A, Pisignano D, Parodi M, Quarto R, Scaglione S (2011) Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 6:e26211. https://doi.org/10.1371/journal.pone.0026211
Qi Y, Mcalpine MC (2010) Nanotechnology-enabled flexible and biocompatible energy harvesting. Energy Environ Sci 3:1275–1285. https://doi.org/10.1039/C0EE00137F
Qian Y, Cheng Y, Song J, Xu Y, Yuan WE, Fan C, Zheng X (2020) Mechano-informed biomimetic polymer scaffolds by incorporating self-powered zinc oxide nanogenerators enhance motor recovery and neural function. Small 16:2000796. https://doi.org/10.1002/smll.202000796
Rajabi AH, Jaffe M, Arinzeh TL (2015) Piezoelectric materials for tissue regeneration: a review. Acta Biomater 24:12–23. https://doi.org/10.1016/j.actbio.2015.07.010
Ramirez-Valles EG, Rodriguez-Pulido A, Barraza-Salas M, Martinez-Velis I, Meneses-Morales I, Ayala-Garcia VM, Alba-Fierro CA (2020) A quest for new cancer diagnosis, prognosis and prediction biomarkers and their use in biosensors development. Technol Cancer Res Treat 19:1533033820957033. https://doi.org/10.1177/1533033820957033
Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S (2012) Advances in polymeric systems for tissue engineering and biomedical applications. Macromol Biosci 12:286–311. https://doi.org/10.1002/mabi.201100325
Reilly P (2007) The impact of neurotrauma on society: an international perspective. Prog Brain Res 161:3–9. https://doi.org/10.1016/S0079-6123(06)61001-7
Ribeiro C, Sencadas V, Correia DM, Lanceros-Mendez S (2015) Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf, B 136:46–55. https://doi.org/10.1016/j.colsurfb.2015.08.043
Richter H, Torres FG, Sanchez J (2008) Strain sensing with a piezoelectric biopolymer. World Forum on Smart Materials and Smart Structures Technology: Proceedings of SMSST'07, World Forum on Smart Materials and Smart Structures Technology (SMSST'07), China, 22–27 May, 2007. CRC Press, p 444. https://doi.org/10.1201/9781439828441
Rodel J, Jo W, Seifert KT, Anton EM, Granzow T, Damjanovic D (2009) Perspective on the development of lead-free piezoceramics. J Am Ceram Soc 92:1153–1177. https://doi.org/10.1111/j.1551-2916.2009.03061.x
Roy D, Cambre JN, Sumerlin BS (2010) Future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci 35:278–301. https://doi.org/10.1016/j.progpolymsci.2009.10.008
Ruan L, Yao X, Chang Y, Zou L, Qin G, Zhang X (2018) Properties and applications of the β phase poly (vinylidene fluoride). Polymers 10:228. https://doi.org/10.3390/polym10030228
Rubiano AM, Carney N, Chesnut R, Puyana JC (2015) Global neurotrauma research challenges and opportunities. Nature 527:S193–S197. https://doi.org/10.1038/nature16035
Saigusa Y (2017) Quartz-based piezoelectric materials. Elsevier, Advanced Piezoelectric Materials. https://doi.org/10.1016/B978-0-08-102135-4.00005-9
Sappati KK, Bhadra S (2018) Piezoelectric polymer and paper substrates: a review. Sensors 18:3605. https://doi.org/10.3390/s18113605
Schmid RD, Scheller F (1989) Biosensors-applications in medicine, environmental protection and process control
Seil JT, Webster TJ (2008) Decreased astroglial cell adhesion and proliferation on zinc oxide nanoparticle polyurethane composites. Int J Nanomed 3:523. https://doi.org/10.2147/ijn.s4346
Senior KR (2010) Bone and muscle: structure, force, and motion. The Rosen Publishing Group, Inc
Shahinpoor M, Kim KJ, Mojarrad M (2007) Artificial muscles: applications of advanced polymeric nanocomposites. CRC Press. https://doi.org/10.1201/9781584887140
Shang Y, Wang H, Chen Z, Zhang X, Rong F, Zhao Y (2019) Porous polyvinylidene fluoride thin-film sensors from colloidal crystal templates. J Nanosci Nanotechnol 19:8104–8111. https://doi.org/10.1166/jnn.2019.16765
Silva C, Pinheiro A, Figueiro S, Goes J, Sasaki J, Miranda M, Sombra A (2002) Piezoelectric properties of collagen-nanocrystalline hydroxyapatite composites. J Mater Sci 37:2061–2070. https://doi.org/10.1023/A:1015219800490
Singh I (1978) The architecture of cancellous bone. J Anat 127:305
Smith M, Kar-Narayan S (2022) Piezoelectric polymers: theory, challenges and opportunities. Int Mater Rev 67:65–88. https://doi.org/10.1080/09506608.2021.1915935
Soin N, Anand S, Shah T (2016) Energy harvesting and storage textiles. Elsevier, Handbook of Technical Textiles. https://doi.org/10.1016/B978-1-78242-465-9.00012-4
Sponchioni M, Palmiero UC, Moscatelli D (2019) Thermo-responsive polymers: applications of smart materials in drug delivery and tissue engineering. Mater Sci Eng, C 102:589–605. https://doi.org/10.1016/j.msec.2019.04.069
Stapleton A, Noor MR, Soulimane T, Tofail SA (2016) Physiological role of piezoelectricity in biological building blocks. World Scientific, Electrically active materials for medical devices. https://doi.org/10.1142/9781783269877_0017
Starr MB, Wang X (2015) Coupling of piezoelectric effect with electrochemical processes. Nano Energy 14:296–311. https://doi.org/10.1016/j.nanoen.2015.01.035
Szabo TL (2004) Diagnostic ultrasound imaging: inside out. Academic press. https://doi.org/10.1016/C2011-0-07261-7
Tanaka T (1982) Piezoelectric devices in Japan. Ferroelectrics 40:167–187. https://doi.org/10.1080/00150198208218168
Tanaka Y, Nakayamada S, Okada Y (2005) Osteoblasts and osteoclasts in bone remodeling and inflammation. Current Drug Targets-Inflammation Allergy 4:325–328. https://doi.org/10.2174/1568010054022015
Tang Y, Chen L, Duan Z, Zhao K, Wu Z (2020) Graphene/barium titanate/polymethyl methacrylate bio-piezoelectric composites for biomedical application. Ceram Int 46:6567–6574. https://doi.org/10.1016/j.ceramint.2019.11.142
Tauer K (2007) Polymer-dispersionen. Max-Planck-Institut
Telega JJ, Wojnar R (2002) Piezoelectric effects in biological tissues. J Theor Appl Mech 40:723–759
Thomas, S., Balakrishnan, P. Sadasivan, SM. 2018. Fundamental biomaterials: ceramics, Woodhead Publishing.
Thuau D, Aabbas M, Wantz G, Hirsch L, Dufour I, Ayela C (2016) Piezoelectric polymer gated OFET: cutting-edge electro-mechanical transducer for organic MEMS-based sensors. Sci Rep 6:1–8. https://doi.org/10.1038/srep38672
Tichy J, Erhart J, Kittinger E, Privratska J (2010) Fundamentals of piezoelectric sensorics: mechanical, dielectric, and thermodynamical properties of piezoelectric materials. Springer Science & Business Media. https://doi.org/10.1007/978-3-540-68427-5
Turner AP (2013) Biosensors: sense and sensibility. Chem Soc Rev 42:3184–3196. https://doi.org/10.1039/C3CS35528D
Tzou H, Lee H-J, Arnold S (2004) Smart materials, precision sensors/actuators, smart structures, and structronic systems. Mech Adv Mater Struct 11:367–393. https://doi.org/10.1080/15376490490451552
Uchino K (2017) Advanced piezoelectric materials: Science and technology. Woodhead Publishing
Vasquez Sancho F (2018) Flexoelectricity in biomaterials. Universitat Autònoma de Barcelona
Vijatovic M, Bobic J, Stojanovic B (2008) History and challenges of barium titanate: Part I. Sci Sinter 40:155–165. https://doi.org/10.2298/SOS0802155V
Vijatovic M, Bobic J, Stojanovic B (2008) History and challenges of barium titanate: Part II. Sci Sinter 40:235–244. https://doi.org/10.2298/SOS0803235V
Vijaya M (2012) Piezoelectric materials and devices: applications in engineering and medical sciences. CRC Press
Wadley HN (1996) Characteristics and processing of smart materials. In Virginia Univ, Smart Structures and Materials: Implications for Military Aircraft of New Generation p (SEE N 97–11475 01–23)
Wang L (2017a) Early Diagnosis of Breast Cancer Sensors 17:1572. https://doi.org/10.3390/s17071572
Wang Q (2016) Smart materials for tissue engineering: fundamental principles. Royal Society of Chemistry. https://doi.org/10.1039/9781782626756
Wang Q (2017) Smart materials for tissue engineering: applications. Royal Society of Chemistry. https://doi.org/10.1039/9781788010542
Wang Q, Yang J, Zhang W, Khoie R, Li YM, Zhu JG, Chen ZQ (2009) Manufacture and cytotoxicity of a lead-free piezoelectric ceramic as a bone substitute—consolidation of porous lithium sodium potassium niobate by Cold Isostatic Pressing. Int J Oral Sci 1:99–104. https://doi.org/10.4248/ijos.09005
Wang Y, Shi Y, Narita F (2021) Design and finite element simulation of metal-core piezoelectric fiber/epoxy matrix composites for virus detection. Sens Actuators, A 327:112742. https://doi.org/10.1016/j.sna.2021.112742
ZL (2009) ZnO nanowire and nanobelt platform for nanotechnology. Mater Sci Eng R Rep 64:33–71. https://doi.org/10.1016/j.mser.2009.02.001
Wang ZL, Kong XY, Ding Y, Gao P, Hughes WL, Yang R, Zhang Y (2004) Semiconducting and piezoelectric oxide nanostructures induced by polar surfaces. Adv Func Mater 14:943–956. https://doi.org/10.1002/adfm.200400180
Wang, ZL. Liu, Y. 2012. Piezoelectric effect at nanoscale. In: BHUSHAN, B. (ed.) Encyclopedia of nanotechnology. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-90-481-9751-4_273
Wang ZL, Song J (2006) Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312:242–246. https://doi.org/10.1126/science.112400
Wojnar R (2012) Piezoelectric phenomena in biological tissues. Springer, Piezoelectric nanomaterials for biomedical applications. https://doi.org/10.1007/978-3-642-28044-3_6
Wolff J (1892) Das Gesetz der Transformation der Knochen, Berlin, A. Law Bone Remodeling, Hirchwild. https://doi.org/10.1055/s-0028-1144106
Xu Q, Gao X, Zhao S, Liu YN, Zhang D, Zhou K, Khanbareh H, Chen W, Zhang Y, Bowen C (2021a) Construction of bio-piezoelectric platforms: from structures and synthesis to applications. Adv Mater 33:2008452. https://doi.org/10.1002/adma.202008452
Xu Q, Gao X, Zhao S, Liu YN, Zhang D, Zhou K, Khanbareh H, Chen W, Zhang Y, Bowen C (2021b) Construction of bio‐piezoelectric platforms: from structures and synthesis to applications. Adv Mater2008452. https://doi.org/10.1002/adma.202170206
Xu X, Chung Y, Brooks AD, Shih W-H, Shih WY (2016) Development of array piezoelectric fingers towards in vivo breast tumor detection. Rev Sci Instrum 87:124301. https://doi.org/10.1063/1.4971325
Xu X, Gifford-Hollingsworth C, Sensenig R, Shih W-H, Shih WYB, AD. (2013) Breast tumor detection using piezoelectric fingers: first clinical report. J Am Coll Surg 216:1168–1173. https://doi.org/10.1016/j.jamcollsurg.2013.02.022
Xue J, Wan D, Lee SE, Wang J (1999) Mechanochemical synthesis of lead zirconate titanate from mixed oxides. J Am Ceram Soc 82:1687–1692. https://doi.org/10.1111/j.1151-2916.1999.tb01987.x
Xue J, Wan D, Wang J (1999) Mechanochemical synthesis of nanosized lead titanate powders from mixed oxides. Mater Lett 39:364–369. https://doi.org/10.1016/S0167-577X(99)00036-1
Ye Z-G (2008) Handbook of advanced dielectric, piezoelectric and ferroelectric materials: synthesis, properties and applications. Elsevier
Zaszczynska A, Sajkiewicz P, Gradys A (2020) Piezoelectric scaffolds as smart materials for neural tissue engineering. Polymers 12:161. https://doi.org/10.3390/polym12010161
Zec H, Shin DJW, T-H. (2014) Novel droplet platforms for the detection of disease biomarkers. Expert Rev Mol Diagn 14:787–801. https://doi.org/10.1586/14737159.2014.945437
Zhang S, Li F, Yu F, Jiang X, Lee H-Y, Luo J, Shrout J (2018) Recent developments in piezoelectric crystals. J Korean Ceram Soc 55:419–439. https://doi.org/10.4191/kcers.2018.55.5.12
Zhang Y, Chen L, Zeng J, Zhou K, Zhang D (2014) Aligned porous barium titanate/hydroxyapatite composites with high piezoelectric coefficients for bone tissue engineering. Mater Sci Eng, C 39:143–149. https://doi.org/10.1016/j.msec.2014.02.022
Zhou H, Lee J (2011) Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater 7:2769–2781. https://doi.org/10.1016/j.actbio.2011.03.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Consent received. The author affirms that Wiley global permissions provided informed consent for publication of the images in Fig. 3.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamel, N.A. Bio-piezoelectricity: fundamentals and applications in tissue engineering and regenerative medicine. Biophys Rev 14, 717–733 (2022). https://doi.org/10.1007/s12551-022-00969-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-022-00969-z