Abstract
Discovery of parasitengone mites (Acari) in the Gulf of Gdańsk deposits of Baltic amber (“Blue Earth” sediment) resulted in the first description of a fossil representative of Microtrombidiidae. The new species, based on larvae, displays affinity to recent members of Montenegtrombium Saboori and Pešić, 2006, Persianthrombium Sedghi, Saboori and Hakimitabar (in Sedghi et al. 2010) and Porttrombidium Haitlinger, 2000, known from the southwestern Palaearctic. A comparison with related genera and species places the newly described taxon in Porttrombidium (as Porttrombidium gedanense sp. nov.). Montenegtrombium is regarded as a junior synonym of Porttrombidium.
Kurzfassung
Die Entdeckung von Milben aus der Gruppe der Parasitengona (Acari) in Baltischem Bernstein aus der “Blauen Erde” der Danziger Bucht führt zur ersten Beschreibung eines fossilen Vertreters der Microtrombidiidae. Die Milbe ist als Larve konserviert und ähnelt rezenten Arten der Gattungen Montenegtrombium Saboori & Pešić, 2006, Persianthrombium Sedghi, Saboori & Hakimitabar (in Sedghi et al. 2010) und Porttrombidium Haitlinger, 2000, alle bekannt aus der südwestlichen Paläarktis. Ein Vergleich mit Arten dieser Gattungen zeigt, dass die neu nachgewiesene Bernstein-Larve als neue Art von Porttrombidium zu betrachten ist – Porttrombidium gedanense sp. nov. Bei der Gattung Montenegtrombium handelt es sich um ein jüngeres Synonym von Porttrombidium.
Similar content being viewed by others
Introduction
Baltic amber, also known as succinite, is widely distributed in central-eastern Europe. The richest and the oldest deposits are located within the Gulf of Gdańsk, at the mouth of the hypothetical Eridanus river, which brought the resin from the primary amber forest (Weitschat and Wichard 2002).
Research on amber inclusions has a history of more than 200 years (Perkovsky et al. 2007; Szwedo and Sontag 2009). Recent studies on the taxonomic grouping of zooinclusions in randomly selected pieces of Baltic amber revealed that mites, constituting more than 20 % of all zooinclusions, are one of the best represented groups in succinite, giving precedence only to Diptera, which account for ca. 40 % of inclusions (Sontag 2003). Despite the abundance of mites in Baltic amber, the knowledge of Eocene acarofauna is scant.
The cohort Parasitengona, comprising ca. 11,000 of the species described to date (ca. 5,000 terrestrial and ca. 6,000 aquatic), constitutes one of the most speciose groups of mites, with relatively scarce knowledge of fossil taxa. The first mention of terrestrial parasitengone amber inclusion originates from 1845 (Berendt 1845; Dunlop et al. 2015; Judson 2012), and relatively few species have been described till now (Dunlop et al. 2015; Konikiewicz and Mąkol 2014; Bartel et al. 2015). A summary of hitherto knowledge of fossil terrestrial parasitengones has been recently provided by Bartel et al (2015). The present work describes Porttrombidium gedanense sp. nov., based on larvae. It is the first representative of Microtrombidiidae found in the fossil record.
Materials and methods
The samples belong to the Museum of Amber Inclusions (MAI), University of Gdańsk, Poland. Representatives of terrestrial Parasitengona mites were found in lumps of Baltic amber (reg. no. MAI 896, MAI 1343, MAI 3048) originating from the Gulf of Gdańsk deposits (incl. MAI 896 and MAI 1343: Sambia Peninsula, Kaliningrad Oblast, Russia). The lumps contained syninclusions (MAI 896: Homoptera; MAI 1343: Diptera: Dolichopodidae and Cecidomyiidae; MAI 3048: Staphylinidae: Pselaphinae (det. Daniel Kubisz), Coleoptera, Arachnida: Araneae, Myriapoda: Chilopoda).
In sample preparation we followed the protocol provided by Sidorchuk (2013). The amber pieces containing inclusions were pre-cut to the following dimensions (mm): 3.3 × 2.6 × 0.1 (MAI 896), 3.0 × 2.0 × 0.1 (MAI 1343) and 2.9 × 2.6 × 0.1 (MAI 3048), using a Dremel 300 rotary tool with a flexible drive and diamond disc. After polishing with a portable USB-powered MiniPolly2 polishing machine, the pieces were placed in cavity slides in thymol and distilled water solution and sealed with a cover glass. The inclusions were examined using a Nikon Eclipse E-600 light microscope, equipped with a DIC, drawing tube, and DS-Fi1 camera, at magnifications of 400× and 1000×. Raw drawings were graphically processed with the GIMP software, whereas the in-focus images were produced using CombineZP software. Measurements are given in micrometers. The samples are stored in Eppendorf vials filled with a solution of thymol and distilled water. The terminology follows Wohltmann et al. (2007) and Mąkol et al. (2014). For the purpose of comparison the type material of the following recent species was studied: Porttrombidium sebastiani Haitlinger, 2000 (holotype) and Montenegtrombium baloutchi Masoumi, Saboori and Seiedy, 2016 (four paratypes: MP 1256) deposited at the Museum of Natural History, University of Wrocław, Poland.
Systematic palaeontology
Class Arachnida Cuvier, 1812
Superorder Actinotrichida Grandjean in van der Hammen, 1961
Order Trombidiformes Reuter, 1909
Suborder Prostigmata Kramer, 1877
Cohort Parasitengona Oudemans, 1909
Family Microtrombidiidae Thor, 1935
Genus Porttrombidium Haitlinger, 2000
Montenegtrombium Saboori and Pešić, 2006, syn. nov.
Type species Porttrombidium sebastiani Haitlinger, 2000, Recent, from Calliptamus italicus (L.) (Orthoptera) collected in Aire de Maire nr Fatima, Portugal.
Diagnosis larva. Microtrombidiinae with three unpaired idiosomal sclerites (scutum, scutellum, and postscutellum). Stolascutum absent. One pair of normal setae (c 1) on scutellum and on postscutellum (d 1). Stephanostome present. Hypostomalae simple. Palp femur with one seta, palp genu with 0–1 setae. Tarsi I–III with two claws and claw-like empodium, inner claw on tarsus III reduced to ca. 1/4 length of the outer claw. Additionally, elongated sword-like seta, similar in length to outer claw, present at tarsus III termination. Scopa and lophotrix absent. fCx = 2–2–1. Coxalae simple.
Deutonymph and adult. Not known.
Remarks. During the most recent re-examination of the type specimen of the type species (Porttrombidium sebastiani Haitlinger, 2000) we could observe that simple, setulated hypostomalae are present in the holotype, thus the character should be considered typical for the genus. The scope of differences observed between Porttrombidium and Montenegtrombium, pertaining to the chaetotaxy of the palps and chaetotaxy of tarsi, reflects the intra-generic variation known for other genera, and does not confirm their separate status. Consequently, we regard Montenegtrombium as a synonym of Porttrombidium. Masoumi et al. (2016) provided the verified characteristics of Montenegtrombium milicae Saboori and Pešić, 2006 (here regarded as Porttrombidium milicae) and pointed to the presence of ‘sword-like lophotrix’ in newly described Montenegtrombium baloutchi (here regarded as Porttrombidium baloutchi), whereas a sword-like seta arising at the tarsus III termination, and not being a lophotrix, is observed in the latter species but also in P. gedanense sp. nov. The lophotrix is absent in members of Porttrombidium.
Porttrombidium gedanense sp. nov.
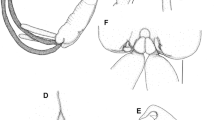
Figures 1, 2, 3, 4, 5, 6, 7 and 8
Etymology. The specific epithet refers to Gdańsk (Latin Gedanum), the Pomeranian city on the Gulf of Gdańsk, hosting the Museum of Amber Inclusions (MAI) (see also Type material) and located ca. 100 km away from amber deposits in the Sambia Peninsula.
Type material. Holotype (no. MAI 1343) and paratypes (no. MAI 896, no. MAI 3048) are deposited at the MAI, Department of Invertebrate Zoology and Parasitology, University of Gdańsk, Poland.
Locality and horizon. Holotype and paratypes originate from the Gulf of Gdańsk sediments (vic. of Jantarny, Sambia Peninsula, Kaliningrad Oblast, Russia), dated at Eocene (Lutetian), ca. 44–50 Ma (Bartel et al. 2015; Weitschat and Wichard 2002).
Diagnosis fnTi = 5–4–4, fnTa = 15–13–10. For other characters, see generic diagnosis.
Description holotype (reg. no. 1343), larva. Habitus as in Fig. 1. Metric data in Table 1.
Gnathosoma (Figs. 2, 3). Stephanostome with horseshoe-like sclerite, devoid of lateral teeth. Hypostomalae (bs) slender, smooth (ca. 17). Palp trochanter and palp genu without setae. Palp femur with one smooth seta (12). On palp tibia only one elongated and smooth seta (37) is visible (see also Remarks). Odontus bifid at termination. Palp tarsus with four short and smooth setae, one slender, elongated and smooth seta and two sensillary setae (?ω and ζ) fPp = 0–N–0–[?][?]N–NNNNN?ωζ.
Idiosoma (Figs. 2, 4). Scutum, scutellum, and postscutellum porous. Scutum longer than wide, slightly incised at the level of ocular plates, with straight posterior margin, with three pairs of non-sensillary setae (AM, AL, PL) and one pair of smooth trichobothria (S). Setae AM arcuately bent, almost smooth. Scutellum with a pair of c 1 setae, postscutellum with d 1 setae. Setae AL, PL, c 1 and d 1 with distinct setules. Remaining setae on idiosoma dorsum, similar in shape to AL, PL, c 1 and d 1, placed on platelets, with stems gradually longer toward the idiosoma termination. Setae h 2 distinctly elongated (101), less than twice as long as h 1 (63). fD = (c 1)c 2–3–(d 1)d 2–3–e 1–3–f 1–3–h 1–2. Ventrally on idiosoma six pseudanal setae, more slender than those covering the idiosoma dorsum. Oval Claparède’s organs between coxae I and II. Setae 1a and 1b—on coxa I, 2a and 2b—on coxa II and 3b—on coxa III. Setae 3a located between coxae III. All coxal and intercoxal setae similar in length, slender and [?] smooth.
Legs (Figs. 5, 6, 7, 8) 6-segmented. Chaetotaxy: leg I—Cx 2n + elc I, Tr 1n, Fe 6n, Ge 4n + 2σ + 1κ, Ti 5n + 2φ + 1κ, Ta 15n + 2ζ + 1ω + 1ε; leg II—Cx 2n, Tr 1n, Fe 5n, Ge 2n + 1σ + 1κ, Ti 4n + 2φ, Ta 13n + 1ζ + 1ω + 1ε; leg III—Cx 1n, Tr 1n, Fe 5n, Ge 2n + 1σ, Ti 4n, Ta 10n. Tarsi I and II terminated with paired claws and claw-like empodium. Tarsus III with modified, markedly reduced inner claw, normally developed outer claw and empodium. Additionally, an elongated sword-like seta present at the Ta III termination.
Remarks the position of the specimen does not allow thorough examination of the chelicerae and setae or within the gnathosoma (the presence of or is typical of parasitengone larvae). Of three setae usually observed on the palp tibia, only one could be detected, due to the blurred surface of the segment. In the area surrounding the anus only six pseudanal setae could be observed (the actual number can depart from this value, however, the multiplication of setae can be excluded).
Comparison the new species differs from Porttrombidium sebastiani Haitlinger, 2000 in the chaetotaxy of tibia and tarsus I–III (fnTi = 5–4–4, fnTa = 15–13–10 in P. gedanense, fnTi = 6–6–6, fnTa = 22–16–17 in P. sebastiani) and in the number of dorsal setae [24 (+4) in P. gedanense, 22 (+4)—in P. sebastiani].
Discussion
Porttrombidium Haitlinger, 2000, originally placed in Trombidiidae, was excluded from the nominate family by Mąkol (2007). Sedghi et al. (2010) placed the genus in Microtrombidiidae, and Mąkol and Wohltmann (2012) treated it as taxon incertae sedis within Microtrombidiinae. Six of the microtrombidiid genera (Cercothrombium Methlagl, 1928, Keramotrombium Southcott, 1994, Persianthrombium Sedghi, Saboori and Hakimitabar, 2010, Porttrombidium Haitlinger, 2000 (=Montenegtrombium Saboori and Pešić, 2006 syn. nov.), Shibadania Southcott, 1994, Workandella Southcott, 1994) have the third, postscutal shield, located medially on the idiosoma dorsum and encompassing the bases of the d 1 setae (Haitlinger 2000; Methlagl 1928; Saboori and Pešić 2006; Sedghi et al. 2010; Southcott 1994). Another genus, Crinitrombium Southcott, 1994, having d 1 plates fused, was synonymised with Microtrombidium by Gabryś and Wohltmann (2001). Of the above mentioned taxa, only Keramotrombium, Persianthrombium, and Porttrombidium (=Montenegtrombium syn. nov.), share the presence of two setae on coxa II and the distinctly reduced inner claw on the tarsus III termination. However, the same combination of characters is observed also in Achaemenothrombiidae Saboori, Wohltmann and Hakimitabar, 2010 and in some representatives of Trombidiidae Leach, 1815, thus the usefulness of these characters for phylogenetic inferences may be limited due to their homoplastic nature.
Keramotrombium Southcott, 1994 was erected in order to accommodate Metathrombium argentanense Bruyant, 1912. Another species, Keramotrombium talebii Karimi Iravanlou and Kamali, 2001, was originally placed in the genus by Karimi Iravanlou and Kamali (2001); however, after the re-appraisal of its characters, the species was transferred to Achaemenothrombium Saboori, Wohltmann and Hakimitabar, 2010. Keramotrombium differs from Porttrombidium also in having the distinct lateral teeth (the latter absent in Porttrombidium) within the stephanostome and in the presence of multiple pseudanal setae (a state not observed in Porttrombidium). Differences between Porttrombidium and Persianthrombium are expressed in the number of solenidia on the genu and tibia I (fsolI = 0–2–2–1 in Porttrombidium, fsolI = 0–4–4–1 in Persianthrombium).
Saboori et al. (2010) erected Achaemenothrombium Saboori, Wohltmann and Hakimitabar, 2010 and a new trombidioid family Achaemenothrombiidae Saboori, Wohltmann and Hakimitabar, 2010, to accommodate two species (among them Keramotrombium talebii Karimi Iravanlou and Kamali, 2001, described from Iran) with a combination of characters not known for any other family level taxon assigned to Trombidioidea: i.e. three dorsal scuta, fCx = 2–2–1, Ti I–III with eight or more normal setae, tibia I with at least four solenidia, multiple solenidia (at least four) and eupathidia (at least six) on tarsus I and multiple solenidia on tarsus II (at least two). The third species assigned to the family was described by Saboori et al (2013). Of the characters of Achaemenothrombiidae, the presence of three dorsal scuta, fCx = 2–2–1, but also the Ta III termination are shared by Keramotrombium, Persianthrombium and Porttrombidium (=Montenegtrombium syn. nov.).
Both P. sebastiani and P. milicae were recorded from Calliptamus italicus (L.) (Orthoptera: Acrididae), P. milicae was recorded also from Carpocoris purpureipennis (De Geer) (Hemiptera: Pentatomidae), whereas P. baloutchi—from Acrida sp., Oedipoda schochii Brunner von Wattenwyl and Chorthippus brunneus (Thunberg) (Orthoptera: Acrididae) (Haitlinger 2000; Masoumi et al. 2016; Saboori and Pešić 2006). Persianthrombium has been hitherto known to parasitize Locusta sp. (Orthoptera: Acrididae) (Sedghi et al. 2010), whereas the host of Keramotrombium remains unknown (Bruyant 1912). Larvae of Achaemenothrombiidae parasitize Lepidoptera and Orthoptera (Saboori et al. 2010, 2013).
The recent species of the above-mentioned genera, tentatively assigned to Microtrombidiidae and of Achaemenothrombiidae have been recorded from Portugal (Porttrombidium), France (Keramotrombium), Montenegro (Montenegtrombium = Porttrombidium), and Iran (Achaemenothrombiidae and Persianthrombium). The discovery of the new fossil member of Porttrombidium from the Gulf of Gdańsk amber deposits may support the hypothesis of similar ecological preferences shared by the recent taxa and inhabitants of the amber forest.
Microtrombidiinae may include genera of heterogeneous origin. A discussion on constructing monophyletic groups (subfamilies) within Microtrombidiidae, supported by a critical review of the literature data, was provided by Wohltmann (2006). The ultimate answer to the question of monophyly of these groups constitutes a crucial point in further conclusions on the phylogeny of subordinate taxa and their position in the system of Microtrombidiidae.
Porttrombidium shares the characters known for Microtrombidiidae (e.g. stephanostome) and for Trombidiidae (e.g. fCx = 2–2–1, termination of tarsus III). The systematic position of Porttrombidium but also of Persianthrombium, and their relationship with other microtrombidiid genera and with Achaemenothrombiidae and Trombidiidae, should be clarified based on further evidence from biology and genetic studies. Discovery of postlarval forms, hitherto unknown both for Porttrombidium, Keramotrombium, Persianthrombium and for Achaemenothrombiidae, may shed a new light on the picture of relationships within Trombidioidea.
References
Bartel, C., M. Konikiewicz, J. Mąkol, A. Wohltmann, and J.A. Dunlop. 2015. Smaridid mites in Baltic and Bitterfeld amber, with notes on the fossil record of terrestrial Parasitengona (Trombidiformes: Prostigmata). Annales Zoologici 65: 641–659.
Berendt, G.C. 1845. Die organischen Bernstein-Einschlüsse im Allgemeinen. In Die im Bernstein befindlichen organischen Reste der Vorwelt gesammelt in Verbindung mit Mehreren bearbeitet und herausgegeben, ed. G.C. Berendt, 41–60. Vol. 1, part I. Berlin: Nicolaische Buchhandlung. http://books.google.com/books?id=mBkGKe4WegAC. Accessed 11 Apr 2015.
Bruyant, L. 1912. Metatrombium argentanense n. sp., une nouvelle larve de Trombidion. Zoologischer Anzeiger 39: 94–96.
Cuvier, G. 1812. Sur un nouveau rapprochment à établir entre les classes qui composant le Règne Animal. Annales du Muséum National d’Histoire Naturelle 19: 73–84.
Dunlop, J.A., D. Penney, and D. Jekel. 2015. A summary list of fossil spiders and their relatives. In World spider catalog. Natural History Museum Bern. Version 16.5. http://wsc.nmbe.ch.
Gabryś, G., and A. Wohltmann. 2001. A redescription of Microtrombidium pusillum (Hermann, 1804) (Acari: Parasitengona: Microtrombidiidae) with notes on taxonomy and biology. Annales Zoologici 51: 233–250.
Haitlinger, R. 2000. A new larval trombidiid, Porttrombidium sebastiani g.nov., n.sp. (Acari: Trombidiidae) parasitic on Calliptamus italicus (L.) (Orthoptera: Catantopidae) from Portugal. Zeszyty Naukowe Akademii Rolniczej we Wrocławiu, Zootechnika 57, 400: 65–68.
Judson, M.L.I. 2012. Status of the family-group names of Arachnida first published in Band I, Abtheilung 1 of Berendt’s Die im Bernstein befindlichen organischen Reste der Vorwelt (1845). Journal of Natural History 46: 1273–1282.
Karimi Iravanlou, J.S., and K. Kamali. 2001. A new larva of the genus Keramotrombium Southcott, 1994 (Acari, Prostigmata, Microtrombidiidae) parasitic on Acrida oxycephala (Pall.) (Orthoptera: Acrididae) from Varamin, Iran. Iranian Journal of Agricultural Sciences 7: 37–47.
Konikiewicz, M., and J. Mąkol. 2014. A fossil Paratrombiinae mite (Actinotrichida: Trombidioidea) from the Rovno amber, Ukraine. Zootaxa 3847: 583–589.
Kramer, P. 1877. Grundzüge zur Systematik der Milben. Archiv für Naturgeschichte 43: 215–247.
Leach, W.E. 1815. A tabular view of the external characters of four classes of animals, which Linné arranged under Insecta; with the distribution of the genera composing three of these classes into Orders, & c., and descriptions of several new genera and species. Transactions of the Linnean Society of London 11: 306–400.
Mąkol, J. 2007. Generic level review and phylogeny of Trombidiidae and Podothrombiidae (Acari: Actinotrichida: Trombidioidea) of the world. Annales Zoologici 57: 1–194.
Mąkol, J., and A. Wohltmann. 2012. An annotated checklist of terrestrial Parasitengona (Actinotrichida: Prostigmata) of the world, excluding Trombiculidae and Walchiidae. Annales Zoologici 62: 359–562.
Mąkol, J., H. Moniuszko, D. Świerczewski, and A. Stroiński. 2014. Planthopper (Hemiptera: Flatidae) parasitized by larval erythraeid mite (Trombidiformes: Erythraeidae)—a description of two new species from Western Madagascar. Journal of Insect Science 14(194): 1–12.
Masoumi, H.R., A. Saboori, and M. Seiedy. 2016. First non-European species of the genus Montenegtrombium (Acari: Microtrombidiidae) ectoparasitic on Acrididae (Orthoptera) from Iran. Systematic and Applied Acarology 21: 288–294.
Methlagl, A. 1928. Über die Trombidiose in den Österreichischen Alpenländern. Denkschriften der Kaiserlichen Akademie der Wissenschaften in Wien, Mathematisch-naturwissenschaftliche Klasse 101(8): 213–250.
Oudemans, A.C. 1909. Über die bis jetzt genauer bekannten Thrombidium-larven und über eine neue Klassifikation der Prostigmata. Tijdschrift Voor Entomologie 52: 19–61.
Perkovsky, E.E., A.P. Rasnitsyn, A.P. Vlaskin, and M.V. Taraschuk. 2007. A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. African Invertebrates 48: 229–245.
Reuter, E. 1909. Zur Morphologie und Ontogenie der Acariden mit besonderer Berucksichtigung von Pediculopsis graminum. Acta Societatis Scientiarum Fennicae 36: 1–288.
Saboori, A., and V. Pešić. 2006. A new genus and species of larval mites (Acari: Microtrombidiidae) from Montenegro. Systematic and Applied Acarology 11: 231–236.
Saboori, A., A. Wohltmann, and M. Hakimitabar. 2010. A new family of trombidioid mites (Acari: Prostigmata) from Iran. Zootaxa 2611: 16–30.
Saboori, A., A. Wohltmann, M. Hakimitabar, and A. Shirvani. 2013. A new species of the genus Achaemenothrombium (Acari: Achaemenothrombiidae) from Iran. Zootaxa 3694: 143–152.
Sedghi, A., A. Saboori, M.A. Akrami, and M. Hakimitabar. 2010. A new genus and species of larval mites (Acari: Prostigmata: Microtrombidiidae) from Iran. Zootaxa 2504: 61–68.
Sidorchuk, K. 2013. A new technique for the preparation of small-sized amber samples with application to mites. In Insect evolution in an amberiferous and stone alphabet, ed. D. Azar, M.S. Engel, E. Jarzembowski, L. Krogmann, A. Nel and J. Santiago-Blay, 189–201. Proceedings of the 6th international congress on fossil insects, arthropods and amber. Leiden: Brill.
Sontag, E. 2003. Animal inclusions in a sample of unselected Baltic amber. Acta Zoologica Cracoviensia 46: 431–440.
Southcott, R.V. 1994. Revision of the larvae of the Microtrombidiinae (Acarina: Microtrombidiidae) with notes on life histories. Zoologica 48(144): 1–155.
Szwedo, J., and E. Sontag. 2009. The traps of the “amber trap”. How inclusions could trap scientists with enigmas. Denisia 26: 155–169. [=Kataloge der Oberösterreichischen Landesmuseen, Neue Serie 86].
Thor, S. 1935. Änderung des Namens einer Unterfamilie der Trombidiidae W.E. Leach 1814. Zoologischer Anzeiger 110(1–2), 1–2: 47.
van der Hammen, L. 1961. Description of Holothyrus grandjeani nov. sp. and notes on the classification of the mites. Nova Guinea, Zoology 9: 173–194.
Weitschat, W., and W. Wichard. 2002. Atlas of plants and animals in Baltic amber. München: Verlag Dr Friedrich Pfeil.
Wohltmann, A. 2006. The phylogenetic relationships of and within the Microtrombidiidae (Acari: Prostigmata: Parasitengona). In Advances in Polish Acarology, ed. G. Gabryś, and S. Ignatowicz, 436–457. Warszawa: Wydawnictwo SGGW.
Wohltmann, A., G. Gabryś, and J. Mąkol. 2007. Acari: terrestrial Parasitengona inhabiting transient biotopes. In Süßwasserfauna von Mitteleuropa 7/2–1, Chelicerata, Araneae, Acari I, ed. R. Gerecke, 158–240. München: Spektrum Elsevier (2006).
Acknowledgments
We are grateful to Dr. Ekaterina A. Sidorchuk who kindly shared her knowledge of amber preparation with us, to Dr. Ryszard Haitlinger who made the type specimen of Porttrombidium sebastiani available for study, and to Dr. Jolanta Jurkowska for the loan of paratypes of Montenegtrombium baloutchi. Our special thanks go to Dr. Jason A. Dunlop and Dr. Andreas Wohltmann for their helpful comments on the manuscript. The work of MK was supported by National Science Centre (Grant in Aid of research 2015/17/N/NZ8/02418).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Konikiewicz, M., Sontag, E. & Mąkol, J. The first description of a microtrombidiid mite (Actinotrichida: Prostigmata, Microtrombidiidae) from Baltic amber, with notes on related extant genera and species. PalZ 90, 493–501 (2016). https://doi.org/10.1007/s12542-016-0311-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-016-0311-y