Abstract
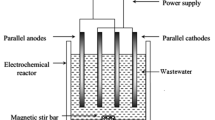
The industrial waste water is considered as a serious threat to environment. Industries with high pollution load of chemical oxygen demand (COD) and biological oxygen demand such as sugar industries has a major role in environmental pollution. The aim of this study was to investigate the treatment possibility of this wastewater by using the electrochemical method. For this purpose quality parameters such as COD, color, turbidity and total dissolved solids (TDS) were investigated. The decrease of color, turbidity COD and TDS from effluent has been investigated at different voltages (10.8, 16.3 and 27.9 V) and various electrolysis times (10, 25 and 40 min) by using different electrodes (Al, Fe) at 4 cm middle distance. Experimental results demonstrated that the electrochemical process by using electrocoagulation and flocculation mechanisms can decrease color, turbidity and COD to 90.8, 98.9 and 50.5 %, respectively. The electrochemical process increased in pH with no considerable effect on the TDS of the effluent by water reclamation on the cathode side. Results showed that electrochemical process without additives could eliminate greatly various containments of wastewater. Therefore, an appropriate method for treating of wastewater can be designed and implemented.







Similar content being viewed by others
References
Aleboyeh, A., N. Daneshvar, and M.B. Kasiri. 2008. Optimization of C.I. acid red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Journal of Chemical Engineering and Processing: Process Intensification 47(5): 827–832.
Anand, V., R. Kandarapu, and S. Garg. 2001. Ion-exchange resins: Carrying drug delivery forward. Drug Discovery Today 6(17): 905–914.
AOAC. 2002. Official methods of analysis, 17th ed. Washington, DC: Association of Official Analytical Chemist. [Method 973.46].
Application information. 1997. Ion exchange resins in the sugar industry. Dow chemical company.
Bejankiwar, R.S., K.S. Lokesh, and T.P. Gowda. 2003. Colour and organic removal of biologically treated coffee curing wastewater by electrochemical oxidation method. Journal of Environmental Sciences 15(3): 323–327.
Bolto, B.A., and L. Pawlowski. 1983. Reclamation of wastewater constituents by ion exchange. Part I. Journal of Effluent Water Treat 23(4): 157–167.
Chen, G. 2004. Electrochemical technologies in wastewater treatment. Journal of Separation and Purification Technology 38(1): 11–41.
Dalvand, A., M. Gholami, A. Joneidi, and N.M. Mahmoodi. 2009. Investigation of electrochemical coagulation process efficiency for removal of reactive red 198 from colored wastewater. Journal of Color Science and Technology 3(2): 97–105. (In Persian).
Daneshvar, N., H. Ashassi Sorkhabi, and M.B. Kasiri. 2004. Decolorization of dye solution containing acid red 14 by electrocoagulation with a comparative investigation of different electrode connections. Journal of Hazardous Materials 112: 55–62.
Do, J.S., M.L. Chen, and Chen. 1994. Decolorization of dye-containing solutions by electrocoagulation. Journal of Applied Electrochemistry 24(8): 785–790.
Eccles, H., and H. Greenwood. 1992. Chklbte ion-exchangers: The past and future application, a user’s view. Journal of Solvent Extraction and Ion Exchange 10(4): 713–727.
Gomes, C.P., M.F. Almeida, and J. M. Loureiro. 2001. Gold recovery with ion exchange used resins. Journal of Separation and Purification Technology 24: 35–57.
Hsing, H.J., P.C. Chiang, E. Chang, and Y. Chen. 2007. The decolorization and mineralization of acid orange 6 azo dye in aqueous solution by advanced oxidation processes. A comparative study. Journal of Hazardous Materials 141(1): 8–16.
ICUMSA. 2000. Methods Book, International commission for uniform methods of sugar analysis, ICUMAS publications, Operations Service, Science. c/o British Sugar plc. [Methods GS2-11, GS7-21, GS2/3-17].
Jiantuan, G., Q. Jiuhui, L. Pengju, and L. Huijuan. 2004. New bipolar electrocoagulation-electro flotation process for treatment of laundry waste water. Journal of Separation and Purification Technology 36(1): 33–39.
Lorimer, J.P., T.J. Mason, M. Plattes, S.S. Phull, and D.J. Walton. 2001. Degradation of dye effluent. Journal of Pure and Applied Chemistry 73(12): 1957–1968.
Mollah, M.Y.A., P. Morkovsky, J.A.G. Gome, M. Kesmez, J. Parga, and D.L. Cocke. 2004. Fundamentals, present and future perspectives of electrocoagulation. Journal of Hazardous Materials 114: 199–210.
Moreira, M.J.A., and L.G.A. Ferreira. 2005. Equilibrium studies of phenylalanine and tyrosine on ion-exchange resins. Journal of Chemical Engineering Science 60(18): 5022–5034.
Phalakornkule, C., S. Polgumhang, and W. Tongdaung. 2009. Performance of an electrocoagulation process in treating direct dye: Batch and continuous up flow processes. Journal of World Academy of Science, Engineering and Technology 57: 28–277.
Sheng, H.L., and C.F. Peng. 1994. Treatment of textile wastewater by electrochemical method. Journal of Water Research 28(2): 277–282.
Vera, E., M. Dornier, J. Ruales, F. Vaillant, and M. Reynes. 2003. Comparison between different ion exchange resins for the deacidification of passion fruit juice. Journal of Food Engineering 57: 199–207.
Vigo, F., L. Avalle and M. Paz. 1983. Disposal of vegetation water from olive oil mills, study of electrochemical oxidation. Journal of Rivista Italiana Delle Sostanze Grasse 60(3): 125–131. (in Italian).
Wilcock, A., J. Tebbens, F. Fuss, J. Wanger, and M. Brewster. 1992. Spectrophotometric analysis of electrochemically treated, simulated, dispersed dyebath effluent. Journal of Texture Chemical Color 24: 29–37.
Yang, C.L., and J. McGarrahan. 2005. Electrochemical coagulation for textile effluent decolorization. Journal of Hazardous Materials 127: 40–47.
Yang, J., J. Chen, Y. Zhou, and W.U. Kangbing. 2011. A nano-copper electrochemical sensor for sensitive detection of chemical oxygen demand. Journal of Sensors and Actuators B: Chemical 153(1): 78–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jahed, E., Khodaparast, M.H.H., Lotfian, F. et al. Performance Investigation of Electrochemical Treatment Process on Wastewater of Applicable Decolorization Resins in Sugar Factories. Sugar Tech 16, 311–318 (2014). https://doi.org/10.1007/s12355-013-0250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-013-0250-9