Abstract
A Japanese woman with a history of Kasai operation for biliary atresia had living-donor liver transplantation at the age of 22. The first episode of refractory HE and late cellular rejection was treated by a high dose of methylprednisolone. The second episode of refractory HE was treated by balloon-occluded retrograde transvenous obliteration for a spleno-renal shunt. However, the third episode of refractory HE occurred 11 years after liver transplantation. The liver cirrhosis and hypersplenism were present with a Child–Pugh score of C-10. Although portal vein flow was hepatopetal, superior mesenteric vein flow regurgitated. We performed proximal total splenic artery embolization (TSAE). Superior mesenteric vein flow changed to a hepatopetal direction and she became clear. At a year after proximal TSAE, her spleen volume had decreased to 589 mL (20% decrease) on computed tomography. She is well and has a Child–Pugh score of 8 without overt HE. We report the first case of refractory HE treated by proximal TSAE that is a possible less invasive treatment option for a selected patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) is a devastating condition. The survival probability of cirrhotic patients experiencing their first episode of HE is 42% at 1 year and 23% at 3 years [1]. HE of end-stage liver disease can be triggered by constipation, dehydration, electrolyte disorder, gastrointestinal bleeding, infection, excessive protein intake, and renal failure [2]. According to the practice guidelines of the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver in 2014, nonabsorbable disaccharides, antibiotics, branched-chain amino acids, l-ornithine, and l-aspartate are used for the treatment of HE patients [3]. When HE is related to an existing portosystemic shunt, occlusion of the shunt is a key therapeutic option [3]. Surgical shunt ligation, embolization of a portosystemic shunt, and balloon-occluded retrograde transvenous obliteration have been reported [4,5,6,7]. Partial splenic artery embolization (PSE) as a supplemental treatment for embolization of a portosystemic shunt with HE has also been reported [8]. Proximal total splenic artery embolization (TSAE) is an effective treatment for splenic artery aneurysms [9]. While there are a few reports on proximal TSAE for the treatment of cirrhotic patients with hypersplenism [10], to the best of our knowledge, there are no reports of proximal TSAE for HE. Here, we report a case of refractory HE treated by proximal TSAE.
Case report
A Japanese woman had a history of Kasai operation for biliary atresia at age of 3 months. Frequent cholangitis developed and liver function deteriorated at age of 22. She was referred to our hospital. Total bilirubin, creatinine, and prothrombin time international normalized ratio were 15.6, 0.45 mg/dL, and 1.41, respectively. The model for end-stage liver disease score was 21. She had ascites, the spleno-renal shunt, and underwent living-donor liver transplantation using her father’s left liver. The post-operative course was uneventful.
The first episode of temporal overt HE occurred 6 years after living-donor liver transplantation. Liver biopsy revealed that her rejection activity index score was 4 (P2, B1, V1) based on the International Banff Schema for Liver Allograft Rejection [11] with focal interportal bridging fibrosis. She was diagnosed with late cellular rejection and treated with a high dose of methylprednisolone and became clear with no other treatment. No portal vein stenosis was detected, and portal vein pressure was 258 mmH2O (19 mmHg) on percutaneous transhepatic portography. HE recurred again 3 years later. The patent spleno-renal shunt was treated by balloon-occluded retrograde transvenous obliteration and she became clear. Treatment with oral nonabsorbable disaccharides was started. Six months later, her esophageal varices ruptured and an endoscopic variceal ligation was performed.
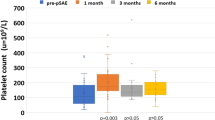
Two years later at the age of 34, she was urgently admitted to our hospital due to recurrent HE, grade 1–3 [3]. Her serum ammonia level was 352 μg/dL. Intravenous administration of branched chain amino acids was started (Fig. 1). Liver cirrhosis and hypersplenism were present with white blood cell and platelet counts at 2400 and 46,000/µL, respectively. Serum albumin, total bilirubin, creatinine, and prothrombin time international normalized ratio were 3.3 g/dL, 1.1 mg/dL, 1.19 mg/dL, and 1.08, respectively. Abdominal ultrasonography revealed massive ascites and hepatopetal portal venous flow. Her Child–Pugh score was 10. Computed tomography showed a hypertrophied splenic artery, 12 mm in diameter. A portosystemic shunt between the umbilical vein and both thick inferior epigastric veins was visualized. There was no portal vein thrombosis. The spleen volume was calculated to be 732 mL using a volume analyzer (Synapse Vincent, Fujifilm Corp., Tokyo, Japan). Superior mesenteric artery angiography also confirmed the presence of a portosystemic shunt via the umbilical vein to bilateral inferior epigastric veins. Celiac artery angiography showed that portal venous flow was hepatopetal, and superior mesenteric venous (SMV) flow regurgitated and hepatofugal (Fig. 2a). Temporary balloon occlusion of the proximal splenic artery was tried and it decreased the hepatic vein wedge pressure from 285 to 231 mmH2O (21 to 17 mmHg) and normalized the SMV flow to the hepatopetal direction. Furthermore, the distal splenic artery was retrogradely visualized via the left gastric artery on the late phase of celiac artery angiography. Since there has been no report in the literature for TSAE reducing portosystemic shunt flow and normalizing the portal venous flow, informed consent was obtained and a proximal TSAE was attempted.
The trend of the patient’s serum ammonia level is indicated by the black line. The solid box indicates overt hepatic encephalopathy (HE) and its grade. The white box indicates the duration of intravenous infusion of a branched chain amino acid (BCAA). The dotted triangle indicates the time of the proximal total splenic artery embolization (proximal TSAE), day 0
Celiac artery angiography revealed the portal vein (PV) and superior mesenteric vein (SMV). Although portal vein flow was hepatopetal, superior mesenteric vein flow regurgitated (a). After proximal total splenic artery embolization (TSAE), superior mesenteric artery angiography showed that SMV flow changed to normal and hepatopetal. The arrows show the direction of each vein flow. Coils were observed in the splenic artery (b)
Cefazolin (1 g) was administered as a prophylactic antibiotic before embolization. In detail, a 20-mm balloon occlusion catheter (Nipro Corp., Osaka, Japan) was placed in the distal splenic artery from the left femoral artery. A 9-mm balloon catheter (Terumo Co., Ltd., Tokyo, Japan) was placed in the proximal splenic artery from the right femoral artery. Both of the balloons were inflated to prevent migration of the coils and 3000 U of heparin was administered. Guglielmi detachable coils (Stryker, Kalamazoo, MI, US), interlocking detachable coils (Boston Scientific, Marlborough, MA, US), and micro coils (Cook, Bloomington, IN, US) were placed in the proximal parts of the splenic artery. At the end of embolization, the distal balloon was deflated and slowly removed while still inflating the proximal splenic artery balloon. Then, the proximal splenic artery balloon was gradually deflated and removed. A final splenic artery angiography was obtained to confirm complete occlusion at the proximal splenic artery. The SMV flow changed to hepatopetal (Fig. 2b). The distal splenic artery was retrogradely visualized via the left gastric artery on the late phase of the post-embolization celiac artery angiography (Fig. 3b, c). Splenic infarction was not observed. The shunt flow between the umbilical vein and both inferior epigastric veins became obscure on superior mesenteric artery angiography. Hepatic vein wedge pressure was not measured. She became clear. Seven days after proximal TSAE, her spleen volume had decreased to 691 mL (6% decrease) on computed tomography. Splenic infarction was not observed.
Celiac artery angiography indicated a hypertrophied splenic artery before embolization (a). Consecutive b (early) and c (early-late) of celiac artery angiography after proximal total splenic artery embolization showed retrogradely visualized distal splenic artery (white arrows) via the left gastric artery
Her serum ammonia level normalized (Fig. 1), renal function was improved, and the amount of ascites decreased. She was discharged from the hospital 9 days after embolization. At 1 year after proximal TSAE, her spleen volume had decreased to 589 mL (20% decrease) on computed tomography. White blood cell and platelet were 4900 and 62,000/µL, respectively. She is well and has a Child–Pugh score of 8 without overt HE.
Discussion
Here, we report the first case of refractory HE treated by proximal TSAE. In the setting of liver transplantation, Herrero and colleagues reported a case of regurgitated SMV flow that changed to normal hepatopetal flow and HE was improved after embolization of a portosystemic shunt at 10 months after liver transplantation [12]. One of our strategies for normalizing hepatofugal SMV flow in the present case was embolization of both the inferior epigastric veins as a portosystemic shunt, but it was judged to be technically too difficult to obstruct both inferior epigastric veins because the procedure needed to obstruct two and more veins at the same time.
Excessive splenic artery flow was another problem. Splenectomy or splenic artery ligation is an invasive procedure for a patient with a Child–Pugh score of 10. Minimally invasive treatment was therefore a suitable strategy for this patient. Splenic artery embolization was reported in 1973 and is a less invasive procedure than surgery [13]. Distal total splenic artery embolization has a risk of severe splenic infarction [14]. Spigos and colleagues reported that PSE is an alternative procedure to reduce portal pressure in cirrhotic patients; however, it may cause post-embolization syndrome, such as splenic abscess and splenic rupture [15]. In the present report, as the patient was treated with immunosuppressants as a transplant recipient, PSE was not selected because of potential difficulties managing post-embolization syndrome and related infection. The late phase of celiac artery angiography was key to predict and avoid splenic infarction. In the present report, we confirmed the distal splenic artery was retrogradely visualized via the left gastric artery during the late phase of celiac artery angiography on test balloon occlusion of the proximal splenic artery.
A relation between the portal vein and splenic artery flow has been reported. The portal vein flow velocity is decreased 16% by clamping the proximal splenic artery in a normal liver patient [16]. Presser and colleagues reported that proximal TSAE after whole-liver transplantation decreases portal vein flow velocity by 46% [17]. Baccarani and colleagues also reported that proximal TSAE significantly reduces portal vein flow velocity by 31% and wedge hepatic vein pressure by 26% after liver transplantation [18]. In the present report, temporary balloon occlusion of the proximal splenic artery decreased the hepatic vein wedge pressure from 285 to 231 mmH2O and changed the SMV flow to hepatopetal. At the same time, although the portosystemic shunts were still patent, the shunt flow between the umbilical vein and both inferior epigastric veins became obscure on superior mesenteric artery angiography which indicates decreased portosystemic shunt flow. Bases on these results, TSAE was performed and hepatic encephalopathy improved.
Proximal TSAE may have an inadequate treatment effect or induce early recurrence of HE. In a non-transplant setting, He and colleagues reported a prospective trial to compare proximal TSAE (n = 27) to PSE (n = 34) in cirrhotic patients with hypersplenism. The proximal TSAE group had a lower complication rate, and better sustained improvement of thrombocytopenia and leukocytopenia for 4 years compared to the PSE group [10]. On the other hand, Yoshida and colleagues reported PSE with portosystemic shunt occlusion for HE (n = 25) in which low ammonia level and low grades of HE were well maintained for 5 years compared to a mono-shunt occlusion group [8]. Although in the present case, the patient was stable for 1 year after proximal TSAE, patients should be carefully followed. It is important to confirm the patency of the collateral arteries of the spleen to avoid severe complications [10], but collateral arteries may also lead to blood flow re-augmentation. Further studies are needed to clarify the optimal procedure for patients with refractory HE and/or regurgitated SMV flow.
Conclusions
Proximal TSAE has the possibility of being a less invasive treatment option for a selected patient with refractory HE.
References
Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–5.
Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21.
Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35.
Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–57.
An J, Kim KW, Han S, et al. Improvement in survival associated with embolisation of spontaneous portosystemic shunt in patients with recurrent hepatic encephalopathy. Aliment Pharmacol Ther. 2014;39(12):1418–26.
Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001;12(3):327–36.
Zidi SH, Zanditenas D, Gelu-Simeon M, et al. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int. 2007;27(10):1389–93.
Yoshida H, Mamada Y, Taniai N, et al. Long-term results of partial splenic artery embolization as supplemental treatment for portal-systemic encephalopathy. Am J Gastroenterol. 2005;100(1):43–7 Epub 2005/01/19.
Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Interv Radiol. 2003;26(3):256–60 (Epub 2003/10/18).
He XH, Gu JJ, Li WT, et al. Comparison of total splenic artery embolization and partial splenic embolization for hypersplenism. World J Gastroenterol WJG. 2012;18(24):3138–44.
Demetris A, Adams D, Bellamy C, et al. Update of the International Banff Schema for liver allograft rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Hepatology. 2000;31(3):792–9 (Epub 2000/03/08).
Herrero JI, Bilbao JI, Diaz ML, et al. Hepatic encephalopathy after liver transplantation in a patient with a normally functioning graft: treatment with embolization of portosystemic collaterals. Liver Transpl. 2009;15(1):111–4.
Maddison FE. Embolic therapy of Hypersplenism. Invest Radiol. 1973;8(4):280–1.
Nussler NC, Settmacher U, Haase R, et al. Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl. 2003;9(6):596–602 (Epub 2003/06/05).
Guan YS, Hu Y. Clinical application of partial splenic embolization. Sci World J. 2014;2014:961345.
Akamatsu N, Sugawara Y, Satou S, et al. Hemodynamic changes in the hepatic circulation after the modulation of the splenic circulation in an in vivo human experimental model. Liver Transpl. 2014;20(1):116–21.
Presser N, Quintini C, Tom C, et al. Safety and efficacy of splenic artery embolization for portal hyperperfusion in liver transplant recipients: a 5-year experience. Liver Transpl. 2015;21(4):435–41.
Baccarani U, Pravisani R, Adani GL, et al. Safety and efficacy of splenic artery embolization for portal hyperperfusion in liver transplant recipients: A 5-year experience. Liver Transpl. 2015;21(11):1457–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Harufumi Maki, Junichi Kaneko, Junichi Arita, Nobuhisa Akamatsu, Yoshihiro Sakamoto, Kiyoshi Hasegawa, Sumihito Tamura, Hidemasa Takao, Eisuke Shibata, and Norihiro Kokudo that they have no conflicts of interest.
Human Rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Maki, H., Kaneko, J., Arita, J. et al. Proximal total splenic artery embolization for refractory hepatic encephalopathy. Clin J Gastroenterol 11, 156–160 (2018). https://doi.org/10.1007/s12328-017-0805-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-017-0805-5