Abstract
Introduction
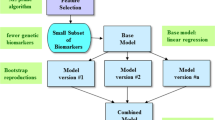
A northern goshawk back-propagation artificial neural network (NGO-BPANN) model was established to predict monohydroxycarbazepine (MHD) concentration in patients with epilepsy.
Methods
The data were collected from 108 Han Chinese patients with epilepsy on oxcarbazepine monotherapy. The results of 14 genotype variates were selected as the input layer in the first BPANN model, and the variables that had a more significant impact on the plasma concentration of MHD were retained. With demographic characteristics and clinical laboratory test results, the genotypes of SCN1A rs2298771 and SCN2A rs17183814 were used to construct the BPANN model. The BPANN model was comprehensively validated and used to predict the MHD plasma concentration of five patients with epilepsy in our hospital.
Results
The model demonstrated favorable fitness metrics, including a mean squared error of 0.00662, a gradient magnitude of 0.00753, an absence of validation tests amounting to zero, and a correlation coefficient of 0.980. Sex, BMI, and the genotype SCN1A rs2298771 were ranked highest by the absolute mean impact value (MIV), which is primarily associated with the concentration of MHD. The test group exhibited a range of − 20.84% to 31.03% bias between the predicted and measured values, with a correlation coefficient of 0.941 between the two. With BPANN, the MHD nadir concentration could be predicted precisely.
Conclusion
The NGO-BPANN model exhibits exceptional predictive capability and can be a practical instrument for forecasting MHD concentration in patients with epilepsy.
Clinical Trial Registration







Similar content being viewed by others
Data Availability
All figures are original and all data and materials included in this study are available upon request by contact with the corresponding author.
References
Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82.
Shorvon S. Oxcarbazepine: a review. Seizure. 2000;9:75–9.
Fisher RS, Cross JH, D’Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–42.
Koch MW, Polman SK. Oxcarbazepine versus carbamazepine monotherapy for partial onset seizures. Cochrane Database Syst Rev. 2009. https://doi.org/10.1002/14651858.CD006453.pub2.
Schmidt D, Elger CE. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav. 2004;5:627–35.
Shaheen U, Prasad DK, Sharma V, et al. Significance of MDR1 gene polymorphism C3435T in predicting drug response in epilepsy. Epilepsy Res. 2014;108:251–6.
Lu Y, Fang Y, Wu X, Ma C, Wang Y, Xu L. Effects of UGT1A9 genetic polymorphisms on monohydroxylated derivative of oxcarbazepine concentrations and oxcarbazepine monotherapeutic efficacy in Chinese patients with epilepsy. Eur J Clin Pharmacol. 2017;73:307–15.
Abe T, Seo T, Ishitsu T, Nakagawa T, Hori M, Nakagawa K. Association between SCN1A polymorphism and carbamazepine-resistant epilepsy. Br J Clin Pharmacol. 2008;66:304–7.
Ma CL, Wu XY, Jiao Z, Hong Z, Wu ZY, Zhong MK. SCN1A, ABCC2 and UGT2B7 gene polymorphisms in association with individualized oxcarbazepine therapy. Pharmacogenomics. 2015;16:347–60.
Thomas SJ, L’Azou M, Barrett ADT, Jackson NAC. Fast-track Zika vaccine development: is it possible? N Engl J Med. 2016;375:1212–6.
van Os HJA, Ramos LA, Hilbert A, et al. Predicting outcome of endovascular treatment for acute ischemic stroke: potential value of machine learning algorithms. Front Neurol. 2018;9:784.
Yamamura S. Clinical application of artificial neural network (ANN) modeling to predict pharmacokinetic parameters of severely ill patients. Adv Drug Deliv Rev. 2003;55:1233–51.
Xu Y, Chen J, Shao R, Ruan Z, Jiang B, Lou H. Development and validation of a new LC–MS/MS method for the determination of mefatinib in human plasma and its first application in pharmacokinetic studies. J Anal Sci Technol. 2022;13:41.
Xu Y, Chen J, Yang D, et al. Development of a particle swarm optimization-backpropagation artificial neural network model and effects of age and gender on pharmacokinetics study of omeprazole enteric-coated tablets in Chinese population. BMC Pharmacol Toxicol. 2022;23:53.
Xu Y, Chen J, Yang D, et al. Development of LC-MS/MS determination method and backpropagation artificial neural networks pharmacokinetic model of febuxostat in healthy subjects. J Clin Pharm Ther. 2021;46:333–42.
Xu Y, Lou H, Chen J, et al. Application of a backpropagation artificial neural network in predicting plasma concentration and pharmacokinetic parameters of oral single-dose rosuvastatin in healthy subjects. Clin Pharmacol Drug Dev. 2020;9:867–75.
Chen J, Xu Y, Lou H, et al. Effect of genetic polymorphisms on the pharmacokinetics of deferasirox in healthy Chinese subjects and an artificial neural networks model for pharmacokinetic prediction. Eur J Drug Metab Pharmacokinet. 2020;45:761–70.
Dehghani M, Hubálovský Š, Trojovský PJIA. Northern goshawk optimization: a new swarm-based algorithm for solving optimization problems. IEEE Access. 2021;9:162059–80.
Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668–79.
Hessler G, Baringhaus KH. Artificial intelligence in drug design. Molecules. 2018;23(10):2520.
Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284:603–19.
Bi Q, Goodman KE, Kaminsky J, Lessler J. What is machine learning? A primer for the epidemiologist. Am J Epidemiol. 2019;188:2222–39.
Medical Writing and Editorial Assistance.
No medical writing or editorial assistance is received during the writing of this article (including AI).
Funding
This study was supported by the National Major Science and Technology Projects of China (No. 2020ZX09201022). The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
The study was designed and conceived by Haibin Dai. The study was conducted by Mingdong Yang, Meng Chen, and Junjun Xu. Data collection, analysis, and model construction were performed by Rong Shao and Yichao Xu. The first draft of the manuscript was written by Yichao Xu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
The study was in accordance with the Declaration of Helsinki, and the studies involving human participants were reviewed and approved by the Human Subject Research Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Shao, R., Yang, M. et al. Application of Northern Goshawk Back-Propagation Artificial Neural Network in the Prediction of Monohydroxycarbazepine Concentration in Patients with Epilepsy. Adv Ther 41, 1450–1461 (2024). https://doi.org/10.1007/s12325-024-02792-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02792-2