Abstract
Introduction
For some immune-mediated disorders, despite the range of therapies available there is limited evidence on which treatment sequences are best for patients and healthcare systems. We investigated how their selection can impact outcomes in an Italian setting.
Methods
A 3-year state-transition treatment-sequencing model calculated potential effectiveness improvements and budget reallocation considerations associated with implementing optimal sequences in ankylosing spondylitis (AS), Crohn’s disease (CD), non-radiographic axial spondyloarthritis (NR-AxSpA), plaque psoriasis (PsO), psoriatic arthritis (PsA), rheumatoid arthritis (RA), and ulcerative colitis (UC). Sequences included three biological or disease-modifying treatments, followed by best supportive care. Disease-specific response measures were selected on the basis of clinical relevance, data availability, and data quality. Efficacy was differentiated between biologic-naïve and experienced populations, where possible, using published network meta-analyses and real-world data. All possible treatment sequences, based on reimbursement as of December 2022 in Italy (analyses’ base country), were simulated.
Results
Sequences with the best outcomes consistently employed the most efficacious therapies earlier in the treatment pathway. Improvements to prescribing practice are possible in all diseases; however, most notable was UC, where the per-patient 3-year average treatment failure was 37.3% higher than optimal. The results focused on the three most crowded and prevalent immunological sub-condition diseases in dermatology, rheumatology, and gastroenterology: PsO, RA, and UC, respectively. By prescribing from within the top 20% of the most efficacious sequences, the model found a 15.1% reduction in treatment failures, with a 1.59% increase in drug costs.
Conclusions
Prescribing more efficacious treatments earlier provides a greater opportunity to improve patient outcomes and minimizes treatment failures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
A 3-year state-transition treatment-sequencing model was developed to calculate potential effectiveness improvements and budget reallocation associated with implementing optimal sequences across seven immunological disease areas. |
The Italian setting was used as the base case for model outcomes, where improvements to current prescribing practices were found to be possible in all diseases, particularly in plaque psoriasis (PsO) and ulcerative colitis (UC). |
What was learned from the study? |
The results indicated that a ‘best treatment first’ approach improved patient outcomes with only a minimal impact on drug costs, especially when budget allocation is leveraged, through the appropriate and efficient use of innovative treatments and biosimilars. |
By altering practice to prescribe from within the top 20% of the most efficacious sequences, the model reported a 15.1% reduction in treatment failures across all diseases, with a 1.59% increase in overall drug costs. |
Introduction
The landscape of immune-mediated disorders includes dermatological, gastrointestinal, and rheumatological diseases. Patients with these disorders can suffer from disabling symptoms and significant complications; there remains significant unmet need for new, effective therapies and evidence for their uptake [1, 2].
Plaque psoriasis (PsO) is a painful, disfiguring, chronic dermatological condition characterized by autoimmune-mediated inflammation of the skin. It typically presents as painful, raised, well-defined, symmetrical, red oval lesions with silvery scales, mostly affecting the elbows, knees, scalp and trunk (although lesions can occur in any region of the body) [3, 4]. It affects between 0.5% and 11.4% of adults worldwide and is associated with other immune-mediated disorders (arthritis, inflammatory bowel disease), as well as depression and cardiovascular diseases [5, 6].
Crohn’s disease (CD) and ulcerative colitis (UC) are both chronic disorders affecting the colonic mucosa and have a prevalence of 0.32% and 0.51% in Europe, respectively [7]. Symptoms include diarrhoea; blood and mucus in the faeces (stools); fever and fatigue; reduced appetite and unintended weight loss; and abdominal pain and cramping, with digestive-tract damage driving disease progression [8, 9]. Patients with a high symptom burden have been shown to be more prone to decreased physical and mental quality of life [10].
Rheumatoid arthritis (RA) has a prevalence of between 0.29% and 0.57% in adults in Europe [11]. Primarily involving the joints, extra-articular manifestations are also common, such as rheumatoid nodules and pulmonary involvement or vasculitis, as well as systemic comorbidities, particularly affecting the vasculature and metabolism [12]. Clinically, people with RA will typically present with a recent onset of tender and swollen joints, morning joint stiffness and generalized sickness symptoms [12].
Psoriatic arthritis (PsA) has a prevalence of between 0.05% and 0.21% in Europe [13]. Symptoms are similar to RA, often involving a similar substantial impairment of physical function (limited dexterity, fatigue, peripheral joint pain/swelling and joint stiffness upon waking). However, PsA is more likely to progress to the tendons, eyes and nails, and more likely to be associated with psoriasis itself [14,15,16]. In this regard, for PsA, control of skin symptoms is often an important measure of a treatment’s effectiveness.
Ankylosing spondylitis (AS, or radiographic axial spondyloarthritis) has a global prevalence of between 0.08% and 0.49% [17, 18] and mainly affects the axial skeleton (particularly the sacroiliac and spinal joints), resulting in severe chronic pain and disability. In advanced disease, patients suffer from ankylosis (the formation of new bone), resulting in the fusion of vertebrae, reduced mobility and long‐term disability. People with AS usually first present with back pain and stiffness between the ages of 20 and 30 years; complications including iritis; an increased risk of osteoporosis and spinal compression fractures; and cardiovascular disease [19].
Non-radiographic axial spondyloarthritis (NR-AxSpA) has been classically considered an early form of AS in patients without structural damage. The proportion of people with AS who have undiagnosed NR-AxSpA is unknown and estimates vary considerably, ranging from 23% to 80% [20]. Symptoms are milder than those associated with radiographic AS, with patients suffering from joint and back pain, but often there is more peripheral involvement with less impaired mobility [21].
Patients with these conditions all require treatment for prolonged periods of their life and finding effective treatments for the duration of their disease can be challenging. The relapsing nature of these diseases means many patients require several different treatments over their lifetime. Whilst multiple new and effective treatment options have become available in recent years, there remains uncertainty around the optimal order or sequence of those treatments. When introduced 20 years ago, anti-tumour necrosis factor-alpha (aTNF) therapies were a step change in the efficacy of treatments available to patients. Since then, numerous newer therapies have become available for patients, raising the bar in terms of what is achievable from a treatment response perspective. While the availability of biologic and disease-modifying anti-rheumatic drugs differs in each disorder, many share the same mechanism of action (MOA). Broad classes of treatments, grouped by their MOA, include anti-integrins, CTLA4-Ig, interleukin (IL)-6, IL-17, IL-23, IL-12/23, Janus kinase inhibitors, phosphodiesterase 4 inhibitors, and aTNF [12, 22,23,24,25].
With the range of treatments available, there are many potential sequences to choose from. Little guidance or evidence is available to clinicians on the optimal sequence for each patient and disorder. Indeed, in current treatment guidelines no recommendations for specific drug sequences are made—after patients have experienced an initial biologic failure, guidelines recommend treating with a drug of either the same or a different MOA [25,26,27]. This lack of specific guidance may not capture the full breadth of informative data that are available to support clinicians in defining or defending their preferred treatment sequence decisions. Furthermore, clinicians may feel their prescribing options are limited by practice budgets, with the use of more costly novel therapies postponed until patients have failed initial cheaper treatments [28, 29]. An additional consideration is the availability and use of biosimilars, which can vary by region and country. With often cheaper acquisition costs, biosimilars can have a significant impact on treatment selection, albeit not necessarily for the most appropriate reasons [30].
A number of studies have attempted to explore or narrow down which sequences of treatments may be more optimal for patient outcomes at a single-disease level, either through analysis of real-world observation data [31, 32], treatment sequencing models [33, 34], or network meta-analyses (NMAs) [35]. However, to date an approach that combines treatment sequence efficacy with local current prescribing practices, and presents the relative impact of cost and benefit associated with adopting the most efficacious sequences across these diseases, has yet to be explored. To find solutions for this clear and recurring unmet need for patients with immune-mediated diseases, and to facilitate discussions with stakeholders including physicians, we developed a model to inform decision-making regarding likely optimal sequences of treatment. We also explored the budget reallocation that could result from the adoption of these alternative sequences, including the impact of continued, but more appropriate, biosimilar usage.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Model Software
The model was developed using the R programming language, with an interactive user interface using the Shiny package [36]. Whilst health economic models are traditionally developed in Microsoft Excel®, there are several advantages to using R as a modelling platform. Modelling in R incorporates direct statistical analysis of datasets, is less prone to functional error through drag-down or copy and paste and provides faster computational times. In addition, Shiny provides a clear and interactive user interface that can be deployed online.
Model Structure
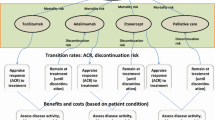
The model used a state-transition approach to track patients across different treatment lines using a 2-week cycle length. To illustrate the full capabilities of this model, the Italian healthcare system perspective was applied. Results were calculated over a 3-year time horizon, which aligns with the decision-making time horizon of most healthcare budget holders. This also limits the uncertainty introduced by the potential arrival of new treatments and the extrapolation of long-term persistency. The start year was 2023. Each sequence included up to three lines of treatments, followed by best supportive care. For PsA, PsO, and RA, as a result of the large number of available treatments with a European Medicines Agency (EMA) label available, a ‘blended’ fourth line of treatment was implemented in the sequence. To model the blended line, the efficacy inputs of all treatments not previously used in the first, second, or third lines were averaged and applied. The transitions of patients through the model are summarized in Fig. 1.
Model structure and data implementation. AS ankylosing spondylitis, CD Crohn’s disease, HTA health technology assessment, NMA network meta-analysis, NR-AxSpA non-radiologic axial spondyloarthritis, PsA psoriatic arthritis, PsO plaque psoriasis, RA rheumatoid arthritis, RWE real-world evidence, UC ulcerative colitis
Patients were assumed to remain on treatment for as long as they responded to therapy. Response outcomes were taken from published trials and NMAs identified in the systematic literature review for all approved therapies; subgroup data by bio-exposure was leveraged where possible as a proxy because true treatment sequencing data remains unavailable for these diseases (e.g. data remains disparate in terms of how prior treatment impacts effectiveness of subsequent treatment). In accordance with previous modelling in immunology, each treatment line is defined by two treatment phases: (i) induction as the starting period of treatment and (ii) a maintenance regimen from the end of the induction period until treatment failure. The clinical significance of these phases differs according to the specific disease; therefore, in alignment with clinical preference and systematic literature review findings, the NMAs for AS, NR-AxSpA, PsA, PsO, and RA are based on response data from the induction period, whilst CD and UC use NMAs informed by maintenance phase response data [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53].
The model assumed that all patients received therapy until induction assessment, as response is traditionally assessed at the end of this phase and reported as such in clinical trials. Next, depending on the NMA type used (i.e. induction NMA or maintenance NMA), patients either discontinue according to the proportion of patients who did not respond at the end of the induction phase or they linearly discontinue until the maintenance assessment point. These differing approaches are presented in Fig. 1. Where an induction NMA is used, discontinuation rates from recently published health technology assessments and assumptions were used to inform the proportion of patients who discontinue between induction assessment and 52 weeks; these were available for NR-AxSpA, PsA, and PsO [54,55,56]. Long-term events after NMA application, or after 52 weeks where discontinuation rates were available, were informed using persistency curves based on real-world evidence [57,58,59,60].
A non-responder event was assumed to be equal to a treatment failure, where the patient would then discontinue and initiate the next line of therapy. If the patient had received all three defined treatment lines and, where relevant, the blended fourth line, the patient was assumed to drop out from biological or disease-modifying treatment and transition to best supportive care, which is an absorbing health state.
Data Inputs and Assumptions
Sources used to inform the model base case were identified through literature reviews and are provided in Table 1. The calculated values per treatment line using these sources are presented in the Supplementary material (Sect. S1). Data were sourced to differentiate between first-line efficacy and efficacy of subsequent lines, with first-line data used across all lines where subsequent line data were unavailable. Whenever possible, the persistency curves were differentiated between diseases and treatments.
The treatment landscape was modelled with options to include any drug with an EMA label in each corresponding disease and treatment line, as of December 2022. The national reimbursement decisions from the Italian Medicines Agency were applied in the model base case to determine which of these treatments were available to patients in each treatment line, and thus the number of possible treatment sequences for each disease [61]. All treatment positioning inputs and the number of sequences generated in the base case are presented in the Supplementary material (Sect. S2).
As the reporting of treatment guidelines in immunology is often linked to the MOA rather than specific treatments, sequences were grouped by MOA so that each is MOA specific, rather than drug specific. Inputs for treatment population size (incident and prevalent) and individual treatment market shares per disease per year were sourced to calculate the total efficacy achieved and costs incurred of the current landscape [62,63,64]. Further inputs included specific biosimilar market shares (relative to originator brands) and the displacement rate of originators by their biosimilars. Market shares, population inputs and biosimilar uptake values for all diseases are available in the Supplementary material (Sect. S2).
The costs considered in the model were the list price drug costs. A summary of key model assumptions is presented in Supplementary material (Sect. S3). To determine the robustness of the model findings scenario analyses tested these data assumptions across all diseases (Sect. S4).
Results Calculations
Each sequence was individually modelled to calculate the respective number of treatment failures and average cost over the 3-year time horizon. The ‘current practice’ calculations estimate the outcomes of the current local clinical practice. To estimate the ‘current practice’ efficacy, sequences were grouped according to first-line therapy; the average number of treatment failures for each sequence was averaged by group. The value of each group was then weighted by incident prescribing market shares to inform the estimated outcome of clinical practice.
A ‘top sequences’ scenario sorts all sequences by average treatment failure number (least to most) and assumes that physicians only prescribe the most efficacious 20% of sequences; it then averages the efficacy equally across these sequences. The percentage is amendable because of the importance in considering varying patient needs, and because physicians need a flexibility in their prescribing; 20% was selected as a compromise between being reasonable for country implementation whilst being impactful.
To estimate the total number of treatment failures, the number of failures for each year of the model is multiplied by the population starting in each year and counted cumulatively. This is done separately for the ‘current practice’ and ‘top sequences’ scenarios to allow direct comparison.
The economic results considered both the incident and total market shares to estimate cumulative costs. The cost of treatment is calculated for each therapy, independent of treatment line, and then weighted by the market shares to produce the average cost for incident patients and prevalent patients for each year. These costs are multiplied by the number of incident and prevalent patients in each year, cumulatively, for the 3-year time horizon. This calculation is taken as the total cumulative cost.
Biosimilar impact is calculated as the difference between the total cumulative cost and the equivalent costs calculated in a scenario, assuming no change in biosimilar uptake (biosimilar displacement rate of 0%) between the start year and final year in the model. The top sequences impact is calculated by using the overall difference in cost between current practice and top sequences practice from the clinical results, and by multiplying by the number of patients per year.
Results
For each disease, all possible sequences were computed to estimate a value for the average number of treatment failures per sequence. Figure 2 shows the variation in efficacy across the disease areas and possible treatment sequences, given as the average number of treatment failures per patient. The range of efficacy is presented by showing the sequence with the lowest average number of treatment failures (green) and that with the highest average number of failures (red) for each condition. Furthermore, the current average prescribing practice, in this case for Italy as determined by market share data, is mapped onto the range (blue triangle) to assess how current practice compares with the possible range of efficacy.
Efficacy variation across disease areas. Lower average number of failures values indicate better outcomes. The values on the lower end (green) of the bars represent the optimal average number of failures for the simulated sequences given the data and treatment positioning for each disease, whilst the values on the upper end (red) represent the highest average number of treatment failures of the sequences simulated. The blue triangle and corresponding value is the calculated current practice average for each disease. AS ankylosing spondylitis, CD Crohn’s disease, NR-AxSpA non-radiologic axial spondyloarthritis, PsA psoriatic arthritis, PsO psoriasis, RA rheumatoid arthritis, UC ulcerative colitis
These results show that the disease with the largest range of patient outcomes (in terms of average number of treatment failures) was PsO whereas CD, AS, and NR-AxSpA all have similarly limited efficacy ranges between the sequences, driven largely by the fact that more treatments are EMA-approved for PsO than for CD, AS, and NR-AxSpA. In assessing Italy’s prescribing practice, the difference between current average prescribing (blue triangle) and the lowest average number of failures per patient (green) illustrates the potential for improvement in selecting treatment sequences with better patient outcomes. The results of the scenario analysis (Sect. S4) showed consistency with the outcomes presented in the base case model.
To further illustrate the capabilities of this model, a deeper assessment was conducted for three of the seven disease areas. PsO, RA, and UC were selected given they are the most crowded and prevalent conditions within the sub-disease categories of dermatology, rheumatology, and gastroenterology, respectively.
The top five MOA sequences with the best efficacy per disease are presented in Table 2. These results show that in all cases, the MOA used in the first line of the most efficacious sequence for each disease matches with the MOA of the therapy with the best relative response from the literature (Sect. S1). Therefore, these results support the use of adopting the most efficacious treatments early in the treatment pathway and, thus, a ‘best treatment first’ approach to improve patient outcomes.
Figure 3 presents the estimated total number of failures over 3 years for both ‘current practice’ and the ‘top sequences’ scenario, assuming the top 20% most efficacious sequences are adopted (Sect. “Results Calculations”). The results suggest that over 3 years, across PsO, RA, and UC, 15.1% of treatment failures could be prevented if the most efficacious sequences were used exclusively in standard clinical practice.
Cumulative number of treatment failures between current practice and the implementation of top sequences for PsO, RA, and UC combined. Lower numbers of failures indicate better patient outcomes. The larger the difference between current practice and top sequence scenarios the larger the opportunity for outcome improvement
Table 3 shows the budget impact of implementing the ‘top sequences’ scenario on the cumulative cost for the full patient population over the 3-year time horizon. This summary also includes the anticipated impact from the uptake of appropriate biosimilar usage from the start year to the end of the model time horizon, which in all cases (where biosimilar usage is not already 100%) contributes to offsetting the cost of increased prescribing of the most efficacious therapies.
For some diseases, there is an increase in the cumulative expenditure if the top sequences are adopted relative to continuing with current practice, while in others, such as RA, switching to prescribing from the top sequences actually decreases overall costs. The total change in drug costs in PsO, RA, and UC, with the implementation of top sequences, is modelled to be + 1.97%. The predicted increase of the natural uptake of biosimilars marginally offsets the impact of switching to the top sequences in some diseases, with the overall impact reducing the top sequences implementation drug costs to + 1.59%.
Discussion
The model was designed to demonstrate the potential improvements in patient outcomes by optimizing treatment sequencing in immunology, prioritising clinical data over limited practice budgets. By incorporating published evidence across multiple indications we have been able to demonstrate the range in efficacy estimates from each possible treatment sequence, and to identify where the biggest improvements in patient outcomes can be made. By incorporating information on treatment costs and modelling the appropriate place in therapy of approved and reimbursed treatments, including biosimilars, we have also been able to calculate the expected resource requirements for making these changes.
Our results highlight that recommending clinicians prescribe more efficacious treatments earlier in the course of therapy provides a greater opportunity to minimize treatment failures and maximize patient outcomes. These findings are supported by other literature, whereby in a single disease area (PsO), modelled treatment sequences starting with more efficacious therapies may have greater health outcomes [33, 34]; our contribution has been to show that current prescribing patterns, often limited by fixed budgets, have potential to improve patient outcomes further. PsO has the largest difference in efficacy between treatment sequences; it is therefore especially important to assess where a country’s prescribing policies and behaviours sit within the wide range of possible outcomes. In the case of Italy, this model helped identify an opportunity for improvement. Whether these results are applicable to other countries will depend on country-level treatment availability and current prescribing practices.
The key advantages of the methodology and approach of this model include the robustness of the inputs, scope of the disease problem explored, and the software used. The model considered the key aspects of immunology care from the perspective of both a patient (treatment response) and payer (treatment response and cost of treatment). This includes the potential sequences of treatments and their financial impact, the current and future market shares, and the efficacy of treatments themselves. By considering multiple diseases across immunology at once, the model totals the potential improvements in patient outcomes that can be made across this area, demonstrating the benefits of making any changes at a practice rather than disease level. Finally, by modelling in R and using the Shiny platform, we can take advantage of the processing power of R; this provides huge benefits when the number of potential treatment sequences increases exponentially (e.g. in PsO, where possible sequence options exceeds 1000).
There are also limitations and areas for improvement in future iterations of this work. One limitation of this study was the availability of cost inputs for the model. Only drug costs and their list prices were considered, given these are publicly available (net price remains confidential between the respective manufactures and the Italian Medicines Agency [AIFA]). While this may inflate the estimated expenditure, an assumption could be made that this inflation would apply consistently across both the ‘current’ and the ‘top sequences’ scenarios. Exploring how the addition of administration costs and healthcare resources (including those related to the management of treatment failures from ineffective treatments) would impact these analyses would also have added value to the work, but these data are not readily available at present. It is likely that consideration of these additional costs, particularly the cost of switching drugs at treatment failure within the induction phase, will further demonstrate the positive impact on outcomes and budget by adopting the top sequences.
As the model considers a number of diseases, it is also likely that new therapies will come to market each year, and as such the number of possible sequences, market landscape, and potential resource impact would change. Given the uncertainty in future treatment approvals and patient access to new therapies, the model is limited in how much of this impact it can capture. A simplifying assumption is made in the analyses that no changes to the market landscape happen over the model time horizon, and that using a time horizon of only 3 years should limit the potential impact of this assumption. Additional information on the duration of time that patients are treatment-free would also be valuable, and allow the assumption that patients start their next line of treatment immediately after failure of the previous line to be relaxed. Nevertheless, the impact of incorporating treatment-free intervals is expected to be negligible over 3 years.
Finally, the model is mostly limited by the lack of specific sequence data in the literature. This means that efficacy data is incorporated directly from trials and real-world data of individual treatments, rather than treatment sequences. Adjustments in efficacy for prior exposure to biologics are made where data are available, but the model does not use ‘true’ sequencing data, where outcomes are based on specific earlier therapies. It is unlikely that such data will be available for all sequences, especially for those including relatively novel treatments, in the near future. The model does, however, make the most of all available evidence and provides a valuable overview of the published evidence of treatment efficacy in these seven immune-mediated diseases. As more real-world data become available, information on the true efficacy of treatment sequences is likely to be discovered. If or when these data become available, it would be valuable to validate the results of this model or inform the model inputs themselves. Indeed, some evidence has already started to come to light within specific diseases that switching MOA is more effective than cycling through therapies with the same MOA [31, 32, 35]. As this evidence base improves, it would be of interest to see if these data influence whether any of the most optimal sequences predicted by our model continue to include those with repeat MOAs. In addition, for many diseases in immunology, disease symptoms can have a significant burden on patient quality of life; the treatments discussed here can resolve that burden to different extents. As well as considering the best sequences for minimizing treatment failures, future studies could look at how minimizing treatment failures through adoption of the best treatment sequences translates to improved patient health-related quality of life.
Conclusion
Our analysis suggests that where reimbursement permits, the MOA used in the first line of the most efficacious sequence for each disease matches the MOA of the therapy with the best response. The results also highlight the limited range in efficacy of available treatment options for particular diseases (CD, AS, and NR-AXSpA), which would highly benefit from additional research and therapeutic candidates.
For this case study, the model shows that the greatest potential improvement in patient outcomes may be achieved in UC and PsO. Over 3 years, across just the three disease areas focused on (PsO, RA, and UC), 15.1% of all treatment failures could be prevented if the 20% most efficacious sequences were used in standard clinical practice in Italy. For some diseases, this is associated with a marginal increase in the cumulative expenditure, and this can be at least partially offset by appropriate biosimilar usage.
This model serves as a valuable tool to understand the difference between current practice and optimal practice with regards to patient outcomes and could assist policymakers and healthcare providers looking to optimize patient health and achieve a sustainable healthcare system within the constraints of fixed healthcare budgets.
Data Availability
All efficacy data used in this study are from published sources with references provided. The Italian market data are included in this article as supplementary information files.
References
Reves J, Ungaro RC, Torres J. Unmet needs in inflammatory bowel disease. Curr Res Pharmacol Drug Discov. 2021;2:100070.
Winthrop KL, Weinblatt ME, Bathon J, et al. Unmet need in rheumatology: reports from the Targeted Therapies meeting 2019. Ann Rheum Dis. 2020;79(1):88–93.
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87(9):626–33.
World Health Organization. Global report on psoriasis. 2016. http://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf. Accessed 13 Dec 2022.
Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20.
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 (e42; quiz e30).
Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17(6):1415–22.
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–70.
Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol. 2002;97(8):1994–9.
Zlatkovic-Svenda MI, Saraux A, Tuncer T, et al. FRI0558 rheumatoid arthritis prevalence in Europe, a Eular-endorsed survey. Ann Rheum Dis. 2016;75(Suppl 2):643.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin N Am. 2015;41(4):545–68.
Giacomelli R, Gorla R, Trotta F, et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxford). 2015;54(5):792–7.
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–70.
Mease P, Strand V, Gladman D. Functional impairment measurement in psoriatic arthritis: importance and challenges. Semin Arthritis Rheum. 2018;48(3):436–48.
Stolwijk C, Boonen A, van Tubergen A, Reveille JD. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am. 2012;38(3):441–76.
Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53(4):650–7.
Voruganti A, Bowness P. New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology. 2020;161(2):94–102.
Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2015;44(5):556–62.
Lopez-Medina C, Ramiro S, van der Heijde D, Sieper J, Dougados M, Molto A. Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open. 2019;5(2):e001108.
Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53(5):585–90.
Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology (Oxford). 2020;59(Suppl 1):i37–46.
Ritchlin C, Adamopoulos IE. Axial spondyloarthritis: new advances in diagnosis and management. BMJ. 2021;372:m4447.
Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34.
Fragoulis GE, Nikiphorou E, Dey M, et al. 2022 EULAR recommendations for screening and prophylaxis of chronic and opportunistic infections in adults with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2023;82(6):742–53.
Dignass A, Dotan I, Lindsay J. Evolving treatment options for patients with ulcerative colitis (UC). 2022. https://www.ecco-ibd.eu/publications/ecco-news/item/2022-1-evolving-treatment-options-for-patients-with-ulcerative-colitis-uc.html. Accessed 30 May 2023.
National Health Service England. Commissioning framework for biological medicines (including biosimilar medicines). 2017. https://www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf. Accessed 13 Dec 2022.
National Institute for Health and Care Excellence. Systemic biological therapy for psoriasis. 2021. https://pathways.nice.org.uk/pathways/psoriasis/systemic-biological-therapy-for-psoriasis.pdf. Accessed 10 Feb 2022.
Vogler S, Schneider P, Zuba M, Busse R, Panteli D. Policies to encourage the use of biosimilars in European countries and their potential impact on pharmaceutical expenditure. Front Pharmacol. 2021;12:625296.
Antolin-Fontes B, Borsi A, Nissinen R, et al. P091 Switching to TNFi versus non-TNFi biologics in Crohn’s disease patients: real-world outcomes from a German claims data analysis. J Crohns Colitis. 2021;15(Suppl_1):S189–91.
Bogas P, Plasencia C, Navarro-Compan V, et al. FRI0079 Long-term clinical efficacy of cycling vs switching to a non-TNF inhibitor in RA patients who failed to a first TNF inhibitor. Ann Rheum Dis. 2019;78(Suppl 2):701.
Egeberg A, Danø A, Pedersen M, Sohrt A, Borg E, Notario J. Modeling the optimal sequence of biologic therapies in plaque psoriasis in Spain. J Med Econ. 2021;24(1):1134–42.
Klijn SL, van den Reek JMPA, van de Wetering G, van der Kolk A, de Jong EMGJ, Kievit W. Biologic treatment sequences for plaque psoriasis: a cost-utility analysis based on 10 years of Dutch real-world evidence from BioCAPTURE. Br J Dermatol. 2018;178(5):1181–9.
Migliore A, Pompilio G, Integlia D, Zhuo J, Alemao E. Cycling of tumor necrosis factor inhibitors versus switching to different mechanism of action therapy in rheumatoid arthritis patients with inadequate response to tumor necrosis factor inhibitors: a Bayesian network meta-analysis. Ther Adv Musculoskelet Dis. 2021;13:2682.
Chang W, Cheng J, Allaire JJ, et al. Shiny. Web Application Framework for R. 2021. https://CRAN.R-project.org/package=shiny. Accessed 23 Oct 2022.
Chen C, Zhang X, Xiao L, Zhang X, Ma X. Comparative effectiveness of biologic therapy regimens for ankylosing spondylitis: a systematic review and a network meta-analysis. Medicine (Baltimore). 2016;95(11):e3060.
Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 study. Ann Rheum Dis. 2017;76(3):571–92.
Committee for Medicinal Products for Human Use (CHMP). Assessment report - Cimzia. Procedure No. EMEA/H/C/001037/II/0065. 26 April 2018. https://www.ema.europa.eu/en/documents/variation-report/cimzia-h-c-1037-ii-0065-epar-assessment-report-variation_en.pdf. Accessed 26 Nov 2019.
Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–60.
Varu A, Wilson FR, Dyrda P, Hazel M, Hutton B, Cameron C. Treatment sequence network meta-analysis in Crohn’s disease: a methodological case study. Curr Med Res Opin. 2019;35(5):733–56.
National Institute for Health and Care Excellence. Golimumab for treating non-radiographic axial spondyloarthritis. 2018. https://www.nice.org.uk/guidance/ta497. Accessed 26 Nov 2019.
Blauvelt A, Papp KA, Griffiths CEM, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17.
Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–31.
Cameron C, Druchok C, Hutton B, et al. Guselkumab for the treatment of moderate-to-severe plaque psoriasis during induction phase: a systematic review and network meta-analysis. J Psoriasis Psoriatic Arthritis. 2019;4(2):81–92.
Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–69.
Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126–36.
Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–25.
Mease PJ, McInnes IB, Tam LS, et al. Comparative effectiveness of guselkumab in psoriatic arthritis: updates to a systematic literature review and network meta-analysis. Rheumatology (Oxford). 2023;62(4):1417–25.
Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67(6):1424–37.
Choy E, Freemantle N, Proudfoot CI, et al. Indirect treatment comparison of the efficacy and safety of sarilumab monotherapy in rheumatoid arthritis patients with inadequate response to conventional disease-modifying antirheumatic drugs. Adv Ther. 2019;36(4):817–27.
Welty M, Mesana L, Padhiar A, et al. Efficacy of ustekinumab vs. advanced therapies for the treatment of moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Curr Med Res Opin. 2020;36(4):595–606.
Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis. 2012;71(7):1134–42.
National Institute for Health and Care Excellence. Secukinumab for treating non-radiographic axial spondyloarthritis (TA719). 2021. https://www.nice.org.uk/guidance/ta719. Accessed 8 Sept 2022.
National institute for Health and Care Excellence. Risankizumab for treating moderate to severe plaque psoriasis (TA596). 2019. https://www.nice.org.uk/guidance/ta596. Accessed 8 Oct 2022.
National Institute for Health and Care Excellence. Guselkumab for treating active psoriatic arthritis after inadequate response to DMARDs (TA711). 2021. https://www.nice.org.uk/guidance/ta711. Accessed 8 Oct 2022.
Jacob L, Chevalier T, Kostev K. Persistence with biological drugs in patients treated in rheumatology practices in Germany. Rheumatol Int. 2019;39(3):525–31.
Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Aliment Pharmacol Ther. 2016;44(7):673–83.
Lunder T, Zorko MS, Kolar NK, et al. Drug survival of biological therapy is showing class effect: updated results from Slovenian National Registry of psoriasis. Int J Dermatol. 2019;58(6):631–41.
Taxonera C, Iglesias E, Munoz F, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior anti-TNF use and efficacy of dose escalation. Dig Dis Sci. 2017;62(2):481–90.
Gazetta Ufficiale. Gazetta Ufficiale della Republica Italiana. 2023. https://www.gazzettaufficiale.it/. Accessed Nov 2022.
IQVIA. Syndicated data: Italian market share and incident / prevalent population data. 2022.
IQVIA. Syndicated data: Italian biosimilar volume data. 2022.
IQVIA. Syndicated data: Italian patient volume data. January 2017–December 2019. 2019.
Acknowledgements
We acknowledge and thank Ottavio Secchi and Pier Cesare Francesa Morel for their contribution in providing the data used to develop the Italian case study presented in this publication.
Funding
The work and the journal’s Rapid Service and Open Access fees are funded by Janssen Cilag.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and analysis of findings were performed by Rose Hart, Karl Patterson, Jennifer Boer, Jennifer Norma Barthelmes, Dawn Lee, Fareen Hassan and Sarah Alulis. The first draft of the manuscript was written by Karl Patterson, Jennifer Boer and Rose Hart, and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Fareen Hassan, Sarah Alulis and Jennifer Norma Barthelmes are employees of Janssen-Cilag with stock or share ownership. Rose Hart, Dawn Lee, Jennifer Boer, and Karl Patterson are/were employees of Lumanity, a company that performs commissioned health-economic related work for several pharmaceutical companies.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hart, R.J., Hassan, F., Alulis, S. et al. Modelling Treatment Sequences in Immunology: Optimizing Patient Outcomes. Adv Ther 41, 2010–2027 (2024). https://doi.org/10.1007/s12325-023-02766-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02766-w