Abstract
Introduction
Amlodipine, valsartan, and rosuvastatin are among the medications widely coadministered for the treatment of hyperlipidemia accompanied by hypertension. The aim of this study was to investigate the possible pharmacokinetic drug–drug interactions between amlodipine, valsartan, and rosuvastatin in healthy Korean male volunteers.
Methods
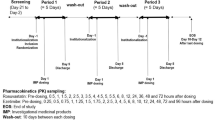
In this phase 1, open-label, multiple-dose, two-part, two-period, fixed-sequence study, the enrolled subjects were randomized into two parts (A and B). In part A (n = 32), each subject received one fixed-dose combination (FDC) tablet of amlodipine/valsartan 10 mg/160 mg alone for 10 consecutive days in period I, and the same FDC for 10 days with concomitant 7-day administration of 20 mg rosuvastatin in period II. In part B (n = 25), each subject received rosuvastatin alone for 7 days in period I, and the FDC for 10 days with concomitant 7-day administration of rosuvastatin in period II. In both parts, there was a 12-day washout between periods. Serial blood samples were collected for up to 72 h for amlodipine and rosuvastatin, and for up to 48 h for valsartan after the last dose of each period. The plasma concentrations of amlodipine, valsartan, and rosuvastatin were determined by using liquid chromatography–tandem mass spectrometry.
Results
Fifty-seven subjects were enrolled; 30 and 25 subjects completed part A and part B, respectively. The geometric mean ratios and 90% confidence intervals for the maximum plasma concentration at steady state (Cmax,ss) and the area under the plasma concentration–time curve over the dosing interval at steady state (AUCτ,ss) were 0.9389 (0.9029–0.9763) and 0.9316 (0.8970–0.9675) for amlodipine, 0.7698 (0.6503–0.9114) and 0.7888 (0.6943–0.8962) for valsartan, and 0.9737 (0.8312–1.1407) and 0.9596 (0.8826–1.0433) for rosuvastatin, respectively. Of the 57 subjects enrolled in this study, 10 subjects experienced 13 adverse events (AEs); no severe or serious AEs were reported.
Conclusion
When amlodipine, valsartan, and rosuvastatin were coadministered to healthy volunteers, the pharmacokinetic exposure to valsartan was decreased, but no change in exposure to amlodipine and rosuvastatin occurred. All treatments were well tolerated.
Clinical Trial Registration
https://cris.nih.go.kr CRIS KCT0001660.
Funding
KyungDong Pharmaceutical Corp. Ltd., Seoul, Republic of Korea.



Similar content being viewed by others
References
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
Egan BM, Li J, Qanungo S, Wolfman TE. Blood pressure and cholesterol control in hypertensive hypercholestrolemic patients: national health and nutrition examination surveys 1988–2010. Circulation. 2013;128:29–41.
Kostis JB. The importance of managing hypertension and dyslipidemia to decrease cardiovascular disease. Cardiovasc Drugs Ther. 2007;21(4):297–309.
Zhang PY. Review of new hypertension guidelines. Eur Rev Med Pharmacol Sci. 2015;19(2):312–5.
James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Rubio-Guerra AF, Castro-Serna D, Barrera CI, Ramos-Brizuela LM. Current concepts in combination therapy for the treatment of hypertension: combined calcium channel blockers and RAAS inhibitors. Integr Blood Press Control. 2009;2:55–62.
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219.
Canbakan B. Rational approaches to the treatment of hypertension: drug therapy-monotherapy, combination, or fixed-dose combination? Kidney Int Suppl (2011). 2013;3(4):349–51.
Pfizer Pharmaceuticals Co. NOVASC drug label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019787s053lbl.pdf. Accessed 20 Aug 2018.
Abernethy DR. The pharmacokinetic profile of amlodipine. Am Heart J. 1989;118(5 Pt 2):1100–3.
Meredity PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet. 1992;22(1):22–31.
Zhu Y, Wang F, Li Q, et al. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab Dispos. 2014;42(2):245–9.
Kim KA, Park PW, Park JY. Effect of ABCB1 (MDR 1) haplotypes derived from G2677T/C3435T on the pharmacokinetics of amlodipine in healthy subjects. Br J Clin Pharmacol. 2007;63(1):53–8.
Zuo XC, Zhang WL, Yuan H, et al. ABCB1 polymorphism and gender affect the pharmacokinetics of amlodipine in Chinese patients with essential hypertension: a population analysis. Drug Metab Pharmacokinet. 2014;29(4):305–11.
Takara K, Matsubara M, Yamamoto K, et al. Differential effects of calcium antagonists on ABCG2/BCRP-mediated drug resistance and transport in SN-38-resistant HeLa cells. Mol Med Rep. 2012;5(3):603–9.
Novartis Pharmaceuticals Co. Diovan drug label. pdf. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021283s035lbl. Accessed 22 Aug 2018.
Zaid AN, Natur S, Qaddomi A, et al. Formulation and bioequivalence of two valsartan/amlodipine immediate release tablets after a single oral administration. Pak J Pharm Sci. 2014;27(4):755–62.
Hanna I, Alexander N, Crouthamel MH, et al. Transport properties of valsartan, sacubitril and its active metabolite (LBQ657) as determinants of disposition. Xenobiotica. 2018;48(3):300–13.
Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—executive summary. J Clin Lipidol. 2014;8(5):473–88.
Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152–60.
Crestor (rosuvastatin calcium) tablets [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021366s030lbl.pdf. Accessed 24 Aug 2018.
Martin PD, Warwick MJ, Dane AL, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25(11):2822–35.
Crouse JR 3rd. An evaluation of rosuvastatin: pharmacokinetics, clinical efficacy and tolerability. Expert Opin Drug Metab Toxicol. 2008;4(3):287–304.
Son J, Guk J, Kim Y, et al. Pharmacokinetic interaction between rosuvastatin, telmisartan, and amlodipine in healthy male Korean subjects: a randomized, open-label, muptiple-dose, 2-period crossover study. Clin Ther. 2016;38(8):1845–57.
Jung JA, Noh YH, Jin S, et al. Pharmacokinetic interaction between pitavastatin and valsartan: a randomized, open-labeled crossover study in healthy male Korean volunteers. Clin Ther. 2012;34(4):958–65.
DeGorter MK, Urquhart BL, Gradhand U, Tirona RG, Kim RB. Disposition of atorvastatin, rosuvastatin, and simvastatin in Oatp1b2–/– mice and intraindividual variability in human subjects. J Clin Pharmacol. 2012;52(11):1689–97.
Ngo L, Cho HY, Lee YB. Effects of hydrochlorthazide and amlodipine on single oral dose pharmacokinetics of valsartan in healthy Korean subjects: population model-based approach. Eur J Pharm Sci. 2018;118:154–64.
Jung JA, Lee SY, Kim JR, et al. A pharmacokinetic and pharmacodynamics drug interaction between rosuvastatin and valsartan in healthy subjects. Drug Des Dev Ther. 2015;9:745–52.
Birmingham BK, Bujac SR, Elsby R, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015;71:329–40.
Acknowledgements
The authors thank all participants of the study.
Funding
This study and article processing charges were sponsored by KyungDong Pharmaceutical Corp. Ltd., Seoul, Republic of Korea, and was supported by Grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2750, HI15C0001), and the Industrial Core Technology Development Program (10051129, Development of the system for ADME assessment using radiolabeled compounds), funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea). All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Vikas Narang of Editage by Cactus Communications Inc. (Trevose, PA, USA), and was funded by KNUH CTC.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Sook Jin Seong, Boram Ohk, Woo Youl Kang, Mi-Ri Gwon, Bo Kyung Kim, Seungil Cho, Dong Heon Yang, Hae Won Lee, and Young-Ran Yoon have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Institutional Review Board at Kyungpook National University Hospital (Daegu, Republic of Korea), and the study was performed in accordance with the ethical standards for studies in humans set out in the Declaration of Helsinki and its amendments, and the applicable guidelines for International Conference on Harmonization Good Clinical Practice, and local laws and regulations. Written informed consent was obtained from all individual subjects included in the study before their enrollment.
Data Availability
The data sets generated and/or analyzed during this study are not publicly available due to confidentiality of KyungDong Pharmaceutical Corp. Ltd., but are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8048198.
Rights and permissions
About this article
Cite this article
Seong, S.J., Ohk, B., Kang, W.Y. et al. Pharmacokinetic Drug Interactions Between Amlodipine, Valsartan, and Rosuvastatin in Healthy Volunteers. Adv Ther 36, 1642–1656 (2019). https://doi.org/10.1007/s12325-019-00976-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00976-9