Abstract
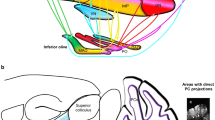
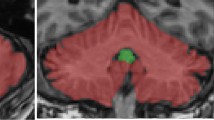
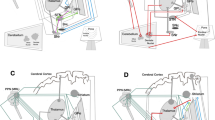
Perception of our linear motion — heading — is critical for postural control, gait, and locomotion, and it is impaired in Parkinson’s disease (PD). Deep brain stimulation (DBS) has variable effects on vestibular heading perception, depending on the location of the electrodes within the subthalamic nucleus (STN). Here, we aimed to find the anatomical correlates of heading perception in PD. Fourteen PD participants with bilateral STN DBS performed a two-alternative forced-choice discrimination task where a motion platform delivered translational forward movements with a heading angle varying between 0 and 30° to the left or to the right with respect to the straight-ahead direction. Using psychometric curves, we derived the heading discrimination threshold angle of each patient from the response data. We created patient-specific DBS models and calculated the percentages of stimulated axonal pathways that are anatomically adjacent to the STN and known to play a major role in vestibular information processing. We performed correlation analyses to investigate the extent of these white matter tracts’ involvement in heading perception. Significant positive correlations were identified between improved heading discrimination for rightward heading and the percentage of activated streamlines of the contralateral hyperdirect, pallido-subthalamic, and subthalamo-pallidal pathways. The hyperdirect pathways are thought to provide top-down control over STN connections to the cerebellum. In addition, STN may also antidromically activate collaterals of hyperdirect pathway that projects to the precerebellar pontine nuclei. In select cases, there was strong activation of the cerebello-thalamic projections, but it was not consistently present in all participants. Large volumetric overlap between the volume of tissue activation and the STN in the left hemisphere positively impacted rightward heading perception. Altogether, the results suggest heavy involvement of basal ganglia cerebellar network in STN-induced modulation of vestibular heading perception in PD.



Similar content being viewed by others
Data Availability
De-identified data will be available to interested parties and will be shared after appropriate institutional agreements are completed.
References
Beylergil SB, Ozinga S, Walker MF, et al. Vestibular heading perception in Parkinson’s disease. Prog Brain Res. 2019;249:307–19.
Yousif N, Bhatt H, Bain PG, et al. The effect of pedunculopontine nucleus deep brain stimulation on postural sway and vestibular perception. Eur J Neurol. 2016;23:668–70. https://doi.org/10.1111/ene.12947.
Bertolini G, Wicki A, Baumann CR, et al. Impaired tilt perception in Parkinson’s disease: a central vestibular integration failure. Plos One. 2015;10:e0124253. https://doi.org/10.1371/journal.pone.0124253.
Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain. 1990;113:767–79. https://doi.org/10.1093/brain/113.3.767.
Beylergil SB, Gupta P, ElKasaby M, et al. Does visuospatial motion perception correlate with coexisting movement disorders in Parkinson’s disease? J Neurol. 2022;269:2179–92.
Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. https://doi.org/10.1056/NEJMoa060281.
Beylergil SB, Noecker AM, Petersen M, et al. Subthalamic deep brain stimulation affects heading perception in Parkinson’s disease. J Neurol. 2022;269:253–68. https://doi.org/10.1007/s00415-021-10616-4.
Rochefort C, Lefort J, Rondi-Reig L (2013) The cerebellum: a new key structure in the navigation system. Front Neural Circuits. Mar 13;7:35.
Kirsch V, Keeser D, Hergenroeder T, et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221:1291–308. https://doi.org/10.1007/s00429-014-0971-x.
Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and Origins. J Neurosci. 2007;27:13590–602. https://doi.org/10.1523/JNEUROSCI.3931-07.2007.
Hill KK, Campbell MC, McNeely ME, et al. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol. 2013;241:105–12. https://doi.org/10.1016/j.expneurol.2012.12.003.
Jenkinson N, Nandi D, Muthusamy K, et al. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009;24:319–28. https://doi.org/10.1002/mds.22189.
Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. NeuroReport. 2005;16:1883–7. https://doi.org/10.1097/01.wnr.0000187637.20771.a0.
Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–70. https://doi.org/10.1007/s11065-010-9143-9.
Moers-Hornikx VMP, Vles JSH, Tan SKH, et al. Cerebellar nuclei are activated by high-frequency stimulation of the subthalamic nucleus. Neurosci Lett. 2011;496:111–5. https://doi.org/10.1016/j.neulet.2011.03.094.
Smith Y, Hazrati L-N, Parent A. Efferent projections of the subthalamic nucleus in the squirrel monkey as studied by the PHA-L anterograde tracing method. J Comp Neurol. 1990;294:306–23. https://doi.org/10.1002/cne.902940213.
Edley SM, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983;217:187–215. https://doi.org/10.1002/cne.902170207.
Ricardo JA. Efferent connections of the subthalamic region in the rat II The zona incerta. Brain Res. 1981;214:43–60. https://doi.org/10.1016/0006-8993(81)90437-6.
Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. https://doi.org/10.1002/cne.903230307.
Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I Retrograde tracing studies. J Comp Neurol. 1992;321:515–43. https://doi.org/10.1002/cne.903210403.
Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–48. https://doi.org/10.1002/cne.903020211.
Giolli RA, Gregory KM, Suzuki DA, et al. Cortical and subcortical afferents to the nucleus reticularis tegmenti pontis and basal pontine nuclei in the macaque monkey. Vis Neurosci. 2001;18:725–40. https://doi.org/10.1017/S0952523801185068.
Liedgren SR, Milne AC, Schwarz DW, Tomlinson RD. Representation of vestibular afferents in somatosensory thalamic nuclei of the squirrel monkey (Saimiri sciureus). J Neurophysiol. 1976;39:601–12. https://doi.org/10.1152/jn.1976.39.3.601.
Hoshi E, Tremblay L, Féger J, et al. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–3. https://doi.org/10.1038/nn1544.
Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol. 2010;68:816–24. https://doi.org/10.1002/ana.22221.
Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. https://doi.org/10.1006/brcg.1999.1099.
Bremmer F, Klam F, Duhamel J-R, et al. Visual–vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci. 2002;16:1569–86. https://doi.org/10.1046/j.1460-9568.2002.02206.x.
Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci. 2007;10:1038–47. https://doi.org/10.1038/nn1935.
Beylergil SB, Petersen M, Gupta P, et al. Severity-dependent effects of Parkinson’s disease on perception of visual and vestibular heading. Mov Disord. 2021;36:360–9.
Noecker AM, Choi KS, Riva-Posse P, et al. StimVision software: examples and applications in subcallosal cingulate deep brain stimulation for depression. Neuromodulation Technol Neural Interface. 2018;21:191–6. https://doi.org/10.1111/ner.12625.
Noecker AM, Frankemolle-Gilbert AM, Howell B, et al. StimVision v2: examples and applications in subthalamic deep brain stimulation for Parkinson’s disease. Neuromodulation Technol Neural Interface. 2021;24:248–58.
Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. https://doi.org/10.1002/mds.22340.
Boutet A, Madhavan R, Elias GJB, et al. Predicting optimal deep brain stimulation parameters for Parkinson’s disease using functional MRI and machine learning. Nat Commun. 2021;12:3043. https://doi.org/10.1038/s41467-021-23311-9.
Guehl D, Cuny E, Benazzouz A, et al. Side-effects of subthalamic stimulation in Parkinson’s disease: clinical evolution and predictive factors. Eur J Neurol. 2006;13:963–71. https://doi.org/10.1111/j.1468-1331.2006.01405.x.
Ravi DK, Baumann CR, Bernasconi E, et al. Does subthalamic deep brain stimulation impact asymmetry and dyscoordination of gait in Parkinson’s disease? Neurorehabil Neural Repair. 2021;35:1020–9. https://doi.org/10.1177/15459683211041309.
Zonenshayn M, Sterio D, Kelly PJ, et al. Location of the active contact within the subthalamic nucleus (STN) in the treatment of idiopathic Parkinson’s disease. Surg Neurol. 2004;62:216–25. https://doi.org/10.1016/j.surneu.2003.09.039.
Ostrem JL, Galifianakis NB, Markun LC, et al. Clinical outcomes of PD patients having bilateral STN DBS using high-field interventional MR-imaging for lead placement. Clin Neurol Neurosurg. 2013;115:708–12. https://doi.org/10.1016/j.clineuro.2012.08.019.
Voges J, Volkmann J, Allert N, et al. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg. 2002;96:269–79. https://doi.org/10.3171/jns.2002.96.2.0269.
Butenko K, van Rienen U. Chapter 7 - DBS imaging methods III: estimating the electric field and volume of tissue activated. In: Horn A, editor. Connectomic Deep Brain Stimulation. Academic Press; 2022. p. 147–68.
Lowery MM (2017) Modeling deep brain stimulation for Parkinson’s disease. In: Computational Models of Brain and Behavior. John Wiley & Sons, Ltd, pp 109–123
Fasano A, Aquino CC, Krauss JK, et al. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11:98–110. https://doi.org/10.1038/nrneurol.2014.252.
Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349:1925–34. https://doi.org/10.1056/NEJMoa035275.
Yamada M, Hoshino M, et al. Precerebellar Nuclei. In: Gruol DL, Koibuchi N, Manto M, et al., editors. Essentials of cerebellum and cerebellar disorders: a primer for graduate students. Cham: Springer International Publishing; 2016. p. 63–7.
Shen L, Jiang C, Hubbard CS, et al. Subthalamic Nucleus Deep Brain Stimulation Modulates 2 Distinct Neurocircuits. Ann Neurol. 2020;88:1178–93. https://doi.org/10.1002/ana.25906.
Wichmann T, DeLong MR. Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics. 2016;13:264–83. https://doi.org/10.1007/s13311-016-0426-6.
Anderson RW, Farokhniaee A, Gunalan K, et al. Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimulat. 2018;11:1140–50. https://doi.org/10.1016/j.brs.2018.05.008.
Miocinovic S, de Hemptinne C, Chen W, et al. Cortical potentials evoked by subthalamic stimulation demonstrate a short latency hyperdirect pathway in humans. J Neurosci Off J Soc Neurosci. 2018;38:9129–41. https://doi.org/10.1523/JNEUROSCI.1327-18.2018.
Walker HC, Huang H, Gonzalez CL, et al. Short latency activation of cortex during clinically effective subthalamic deep brain stimulation for Parkinson’s disease. Mov Disord. 2012;27:864–73. https://doi.org/10.1002/mds.25025.
Sanders TH, Jaeger D. Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol Dis. 2016;95:225–37. https://doi.org/10.1016/j.nbd.2016.07.021.
Bingham CS, McIntyre CC. Subthalamic deep brain stimulation of an anatomically detailed model of the human hyperdirect pathway. J Neurophysiol. 2022;127:1209–20. https://doi.org/10.1152/jn.00004.2022.
Coudé D, Parent A, Parent M. Single-axon tracing of the corticosubthalamic hyperdirect pathway in primates. Brain Struct Funct. 2018;223:3959–73. https://doi.org/10.1007/s00429-018-1726-x.
Pulliam CL, Heldman DA, Brokaw EB, et al. Continuous assessment of levodopa response in Parkinson’s disease using wearable motion sensors. IEEE Trans Biomed Eng. 2018;65:159–64. https://doi.org/10.1109/TBME.2017.2697764.
Evers LJW, Krijthe JH, Meinders MJ, et al. Measuring Parkinson’s disease over time: the real-world within-subject reliability of the MDS-UPDRS. Mov Disord. 2019;34:1480–7. https://doi.org/10.1002/mds.27790.
Acknowledgements
The authors thank Sarah Ozinga, PhD, Palak Gupta, and Mark Walker, MD, for their support.
Funding
This study is funded by grants from US Department of Veterans Affairs Merit Review 5 I01 CX002086-03 (Shaikh), The American Academy of Neurology (Shaikh), American Parkinson’s Disease Association George C Cotzias Memorial Fellowship (Shaikh), Dystonia Medical Research Foundation Research (Shaikh), and philanthropic funds to the Department of Neurology at University Hospitals (The Allan Woll Fund and The Fox Fund). Shaikh also has Penni and Steven Weinberg Chair in Brain Health.
The research was supported by American Academy of Neurology Career Award, George C Cotzias Memorial Fellowship and Department of VA I01 CX002086.
Author information
Authors and Affiliations
Contributions
AS, SB: conceptualized the project, collected data, analyzed data, wrote the manuscript, and edited the manuscript. CM, AN: analyzed data and edited the manuscript. CK: collected data and edited the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Institutional internal review board at University Hospitals Cleveland Medical Center and Cleveland VA Medical Center has approved the study protocol. The participants signed informed consent before participation in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beylergil, S.B., Noecker, A.M., Kilbane, C. et al. Does Vestibular Motion Perception Correlate with Axonal Pathways Stimulated by Subthalamic Deep Brain Stimulation in Parkinson’s Disease?. Cerebellum 23, 554–569 (2024). https://doi.org/10.1007/s12311-023-01576-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-023-01576-8