Abstract
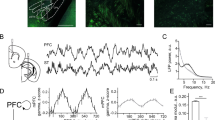
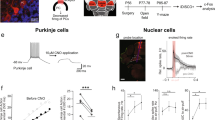
Spatial working memory (SWM) is a cerebrocerebellar cognitive skill supporting survival-relevant behaviors, such as optimizing foraging behavior by remembering recent routes and visited sites. It is known that SWM decision-making in rodents requires the medial prefrontal cortex (mPFC) and dorsal hippocampus. The decision process in SWM tasks carries a specific electrophysiological signature of a brief, decision-related increase in neuronal communication in the form of an increase in the coherence of neuronal theta oscillations (4–12 Hz) between the mPFC and dorsal hippocampus, a finding we replicated here during spontaneous exploration of a plus maze in freely moving mice. We further evaluated SWM decision-related coherence changes within frequency bands above theta. Decision-related coherence increases occurred in seven frequency bands between 4 and 200 Hz and decision-outcome–related differences in coherence modulation occurred within the beta and gamma frequency bands and in higher frequency oscillations up to 130 Hz. With recent evidence that Purkinje cells in the cerebellar lobulus simplex (LS) represent information about the phase and phase differences of gamma oscillations in the mPFC and dorsal hippocampus, we hypothesized that LS might be involved in the modulation of mPFC-hippocampal gamma coherence. We show that optical stimulation of LS significantly impairs SWM performance and decision-related mPFC-dCA1 coherence modulation, providing causal evidence for an involvement of cerebellar LS in SWM decision-making at the behavioral and neuronal level. Our findings suggest that the cerebellum might contribute to SWM decision-making by optimizing the decision-related modulation of mPFC-dCA1 coherence.





Similar content being viewed by others
References
Amemiya S, Redish AD. Hippocampal theta-gamma coupling reflects state-dependent information processing in decision making. Cell Rep. 2018;22:3328–38. https://doi.org/10.1016/j.celrep.2018.02.091.
Eichenbaum H. The role of the hippocampus in navigation is memory. J Neurophysiol. 2017;117:1785–96. https://doi.org/10.1152/jn.00005.2017.
Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res. 2011;225:389–95. https://doi.org/10.1016/j.bbr.2011.07.045.
Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:486–91. https://doi.org/10.1016/j.conb.2011.02.012.
Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 2011;21:475–85. https://doi.org/10.1016/j.conb.2011.01.004.
Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3: e402. https://doi.org/10.1371/journal.pbio.0030402.
Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front Integr Neurosci. 2010;4:2. https://doi.org/10.3389/neuro.07.002.2010.
Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–80.
Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–12. https://doi.org/10.1126/science.1139597.
Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron. 2012;75:875–88. https://doi.org/10.1016/j.neuron.2012.06.037.
Brunet NM, Bosman CA, Vinck M, Roberts M, Oostenveld R, Desimone R, De Weerd P, Fries P. Stimulus repetition modulates gamma-band synchronization in primate visual cortex. Proc Natl Acad Sci U S A. 2014. https://doi.org/10.1073/pnas.1309714111.
McAfee SS, Liu Y, Dhamala M and Heck DH. Thalamocortical transmission of visual information in mice involves synchronized spiking aligned with high gamma oscillations. Front Neurosci 2018: 12. doi https://doi.org/10.3389/fnins.2018.00837
Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–19. https://doi.org/10.1016/j.neuron.2008.09.010.
Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–85.
King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019;22:1371–8. https://doi.org/10.1038/s41593-019-0436-x.
Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE and Wang SS. Normal cognitive and social development require posterior cerebellar activity. eLife 2018: 7. doi https://doi.org/10.7554/eLife.36401
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12.
Watson TC, Obiang P, Torres-Herraez A, Watilliaux A, Coulon P, Rochefort C and Rondi-Reig L. Anatomical and physiological foundations of cerebello-hippocampal interaction. eLife 2019: 8. doi https://doi.org/10.7554/eLife.41896
McAfee S, Liu Y, Sillitoe RV, Heck DH. Cerebellar lobulus simplex and Crus I differentially represent phase and phase difference of prefrontal cortical and hippocampal oscillations. Cell Rep. 2019;27:2328–34. https://doi.org/10.1016/j.celrep.2019.04.085.
Desmond JE, Chen SH, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann Neurol. 2005;58:553–60. https://doi.org/10.1002/ana.20604.
Buzsaki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–188. https://doi.org/10.1002/hipo.22488.
Richman CL, Dember WN, Kim P. Spontaneous alternation behavior in animals: a review. Current Psychological Research & Reviews. 1987;5:358–91.
Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104.
Leske S, Dalal SS. Reducing power line noise in EEG and MEG data via spectrum interpolation. Neuroimage. 2019;189:763–76. https://doi.org/10.1016/j.neuroimage.2019.01.026.
Mewett DT, Reynolds KJ, Nazeran H. Reducing power line interference in digitised electromyogram recordings by spectrum interpolation. Med Biol Eng Compu. 2004;42:524–31. https://doi.org/10.1007/bf02350994.
Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76:691–708. https://doi.org/10.1016/S0006-3495(99)77236-X.
Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–36. https://doi.org/10.1016/j.neuron.2010.05.013.
Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–7. https://doi.org/10.1038/nature08855.
Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–135.
Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352:812–6. https://doi.org/10.1126/science.aad5252.
Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–9. https://doi.org/10.1073/pnas.0913016107.
Lindeman S, Kros L, Hong S, Mejias JF, Romano V, Negrello M, Bosman LWJ and De Zeeuw CI. Cerebellar Purkinje cells can differentially modulate coherence between sensory and motor cortex depending on region and behavior. PNAS 2021: 118. doi https://doi.org/10.1073/pnas.2015292118
Popa D, Spolidoro M, Proville RD, Guyon N, Belliveau L, Lena C. Functional role of the cerebellum in gamma-band synchronization of the sensory and motor cortices. J Neurosci. 2013;33:6552–6. https://doi.org/10.1523/JNEUROSCI.5521-12.2013.
Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. https://doi.org/10.1016/s0306-4522(99)00418-2.
Braitenberg V, Heck DH, Sultan F. The detection and generation of sequences as a key to cerebellar function. Experiments and theory BehavBrain Sci. 1997;20:229–45.
Diener HC, Dichgans J, Guschlbauer B, Bacher M, Rapp H, Klockgether T. The coordination of posture and voluntary movement in patients with cerebellar dysfunction. Mov Disord. 1992;7:14–22.
Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–32.
Raghavan RT, Prevosto V, Sommer MA. Contribution of cerebellar loops to action timing. Curr Opin Behav Sci. 2016;8:28–34. https://doi.org/10.1016/j.cobeha.2016.01.008.
Zacharia TT, Eslinger PJ. Functional MRI activation patterns of cerebellum in patients with epilepsy and brain tumors. Clin Anat. 2019;32:1053–60. https://doi.org/10.1002/ca.23439.
Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66. https://doi.org/10.3389/fpubh.2015.00066.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. https://doi.org/10.1007/s12311-012-0355-9.
McAfee SS, Liu Y, Sillitoe RV and Heck DH. Cerebellar coordination of neuronal communication in cerebral cortex. Frontiers in Systems Neuroscience 2022: 15. doi https://doi.org/10.3389/fnsys.2021.781527
Acknowledgements
We would like to thank the Neuroscience Institute of the University of Tennessee Health Science Center (UTHSC) for the financial support, Micheal Nguyen from the UTHSC Bio-Medical Services and Shuhua Qi for technical support. We would also like to thank Amanda Brown and Trace Stay (Baylor College of Medicine) for the support with some of the breeding and also for genotyping. DHH and YL are supported by R01MH112143, R01MH112143-02S1, and R37MH085726. RVS is supported by R01NS100874, R01NS119301, R01MH112143, and U54HD083092.
Author information
Authors and Affiliations
Contributions
Conceptualization: DHH, SSM, RVS, YL. Formal analysis: YL, SSM, and MD. Funding acquisition: DHH and RVS. Methodology: YL. Supervision: DHH. Writing – original draft: YL and DHH. Writing – review and editing: DHH, YL, SSM, MEvdH, MD, RVS.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., McAfee, S.S., Van Der Heijden, M.E. et al. Causal Evidence for a Role of Cerebellar Lobulus Simplex in Prefrontal-Hippocampal Interaction in Spatial Working Memory Decision-Making. Cerebellum 21, 762–775 (2022). https://doi.org/10.1007/s12311-022-01383-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01383-7