Abstract
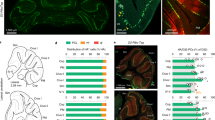
Recent studies suggest that the cerebellum may have a significant role in repetitive behaviors. In primary complex motor stereotypies, typically developing children have repetitive movements usually involving rhythmic flapping/waving arm/hand movements. Similarly, the deer mouse animal model exhibits inherited repetitive behaviors, with increased frequencies of spontaneous jumping and rearing. In this study, data from both children with motor stereotypies and deer mice were used to investigate the role of the cerebellum in repetitive behaviors. The 3.0-T MRI volumetric imaging of the cerebellum was obtained in 20 children with primary complex motor stereotypies and 20 healthy controls. In deer mice, cerebellar volume (n = 7/group) and cell counts (n = 9/group) were compared between high- and low-activity animals. Levels of cerebellar neurotransmitters were also determined via HPLC (n = 10/group). In children with stereotypies, (a) there were a statistically significant reduction (compared to controls) in the white matter volume of the posterior cerebellar lobule VI–VII that negatively correlated with motor control and (b) an 8% increase in the anterior vermis gray matter that positively correlated with motor Stereotypy Severity Scores (SSS). In deer mice, (a) there was a significant increase in the volume of the anterior vermal granular cell layer that was associated with higher activity and (b) dentate nucleus cell counts were higher in high activity animals. Similar increases in volume were observed in anterior vermis in children with stereotypies and a deer mouse model of repetitive behaviors. These preliminary findings support the need for further investigation of the cerebellum in repetitive behaviors.






Similar content being viewed by others
References
Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 2018;19(6):338–50.
Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, Doya K, et al. Consensus paper: Towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum. 2017;16(1):203–29.
Tanaka H, Ishikawa T, Kakei S. Neural evidence of the cerebellum as a state predictor. Cerebellum. 2019;18(3):349–71.
Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68(23):1979–87.
Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(Pt 8):2029–37.
Malone A, Manto M, Hass C. Dissecting the links between cerebellum and dystonia. Cerebellum. 2014;13(6):666–8.
O’Callaghan C, Hornberger M, Balsters JH, Halliday GM, Lewis SJ, Shine JM. Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain. 2016;139(Pt 3):845–55.
Caligiore D, Mannella F, Arbib MA, Baldassarre G. Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLoS Comput Biol. 2017;13(3):e1005395.
Sigurdsson HP, Jackson SR, Jolley L, Mitchell E, Jackson GM. Alterations in cerebellar grey matter structure and covariance networks in young people with Tourette syndrome. Cortex. 2020;126:1–15.
Augustine F, Singer HS. Merging the pathophysiology and pharmacotherapy of tics. Tremor Other Hyperkinet Mov (N Y). 2018;8:595.
Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363(6424):eaav0581. https://doi.org/10.1126/science.aav0581.
Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107(18):8452–6.
Pelzer EA, Hintzen A, Goldau M, von Cramon DY, Timmermann L, Tittgemeyer M. Cerebellar networks with basal ganglia: feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. Eur J Neurosci. 2013;38(8):3106–14.
Singer HS. Motor stereotypies. Semin Pediatr Neurol. 2009;16(2):77–81.
Miller JM, Singer HS, Bridges DD, Waranch HR. Behavioral therapy for treatment of stereotypic movements in nonautistic children. J Child Neurol. 2006;21(2):119–25.
Specht MW, Mahone EM, Kline T, Waranch R, Brabson L, Thompson CB, et al. Efficacy of parent-delivered behavioral therapy for primary complex motor stereotypies. Dev Med Child Neurol. 2017;59(2):168–73.
Singer HS, Rajendran S, Waranch HR, Mahone EM. Home-based, therapist-assisted, therapy for young children with primary complex motor stereotypies. Pediatr Neurol. 2018;85:51–7.
Singer HS. Motor control, habits, complex motor stereotypies, and Tourette syndrome. Ann N Y Acad Sci. 2013;1304:22–31.
Kates WR, Lanham DC, Singer HS. Frontal white matter reductions in healthy males with complex stereotypies. Pediatr Neurol. 2005;32(2):109–12.
Mahone EM, Crocetti D, Tochen L, Kline T, Mostofsky SH, Singer HS. Anomalous putamen volume in children with complex motor stereotypies. Pediatr Neurol. 2016;65:59–63.
Harris AD, Singer HS, Horska A, Kline T, Ryan M, Edden RA, et al. GABA and glutamate in children with primary complex motor stereotypies: an 1H-MRS study at 7T. AJNR Am J Neuroradiol. 2016;37(3):552–7.
Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49(8):655–64.
de Brito Henriques JG, Henriques KS, Filho GP, Fonseca LF, Cardoso F, Da Silva MC. Bobble-head doll syndrome associated with Dandy-Walker syndrome Case report. J Neurosurg. 2007;107(3 Suppl):248–50.
Hottinger-Blanc PM, Ziegler AL, Deonna T. A special type of head stereotypies in children with developmental (?cerebellar) disorder: description of 8 cases and literature review. Eur J Paediatr Neurol. 2002;6(3):143–52.
Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176(1):66–74.
Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008;189(2):250–6.
Bechard AR, Cacodcar N, King MA, Lewis MH. How does environmental enrichment reduce repetitive motor behaviors? Neuronal activation and dendritic morphology in the indirect basal ganglia pathway of a mouse model. Behav Brain Res. 2016;299:122–31.
Bechard AR, Bliznyuk N, Lewis MH. The development of repetitive motor behaviors in deer mice: effects of environmental enrichment, repeated testing, and differential mediation by indirect basal ganglia pathway activation. Dev Psychobiol. 2017;59(3):390–9.
Presti MF, Watson CJ, Kennedy RT, Yang M, Lewis MH. Behavior-related alterations of striatal neurochemistry in a mouse model of stereotyped movement disorder. Pharmacol Biochem Behav. 2004;77(3):501–7.
Korff S, Stein DJ, Harvey BH. Stereotypic behaviour in the deer mouse: pharmacological validation and relevance for obsessive compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):348–55.
Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016(4):pdb.prot087379.
Augustine F, Rajendran S, Singer HS. Cortical endogenous opioids and their role in facilitating repetitive behaviors in deer mice. Behav Brain Res. 2020;379:112317.
Spampinato DA, Celnik PA, Rothwell JC. Cerebellar-motor cortex connectivity: one or two different networks? J Neurosci. 2020;40(21):4230–9.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23(23):8432–44.
Tully HM, Dempsey JC, Ishak GE, Adam MP, Mink JW, Dobyns WB, et al. Persistent figure-eight and side-to-side head shaking is a marker for rhombencephalosynapsis. Mov Disord. 2013;28(14):2019–23.
Dean SL, Huisman T, Poretti A, Singer HS. Figure of eight stereotypies in a young girl with a prenatal cerebellar injury. Mov Disord Clin Pract. 2019;6(6):488–90.
Mahone EM, Ryan M, Ferenc L, Morris-Berry C, Singer HS. Neuropsychological function in children with primary complex motor stereotypies. Dev Med Child Neurol. 2014;56(10):1001–8.
Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20(3):261–70.
Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491–3.
Thach WT, Jones EG. The cerebellar dentatothalamic connection: terminal field, lamellae, rods and somatotopy. Brain Res. 1979;169(1):168–72.
Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci U S A. 2011;108(38):16068–73.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44.
Shafer RL, Newell KM, Lewis MH, Bodfish JW. A cohesive framework for motor stereotypy in typical and atypical development: the role of sensorimotor integration. Front Integr Neurosci. 2017;11:19.
Park IS, Lee NJ, Rhyu IJ. Roles of the declive, folium, and tuber cerebellar vermian lobules in sportspeople. J Clin Neurol. 2018;14(1):1–7.
Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318(21):1349–54.
Bernard JA, Peltier SJ, Benson BL, Wiggins JL, Jaeggi SM, Buschkuehl M, et al. Dissociable functional networks of the human dentate nucleus. Cereb Cortex. 2014;24(8):2151–9.
Manto M, Bower JM, Conforto AB, Delgado-Garcia JM, da Guarda SN, Gerwig M, et al. Consensus paper: Roles of the cerebellum in motor control–the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87.
Wilkes BJ, Bass C, Korah H, Febo M, Lewis MH. Volumetric magnetic resonance and diffusion tensor imaging of C58/J mice: neural correlates of repetitive behavior. Brain Imaging Behav. 2020;14(6):2084–96.
Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, et al. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25(12):3234–45.
Woo J, Min JO, Kang DS, Kim YS, Jung GH, Park HJ, et al. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc Natl Acad Sci U S A. 2018;115(19):5004–9.
Singer HS, Augustine F. Controversies surrounding the pathophysiology of tics. J Child Neurol. 2019;34(13):851–62.
Acknowledgements
We wish to thank Joshua Wilhide, Maggie LaCourse, and all the other staff members of Molecular Characterization and Analysis Complex (MCAC) at the University of Maryland Baltimore County (UMBC) for their superb support in neurochemical quantification process. Thanks to Dr. Nae-Yuh Wang for the statistical expertise. Figure 5 is adapted from SMART-Servier Medical Art and is provided for use free of charge under Creative Commons.
Funding
This work was supported by philanthropic gifts to the Johns Hopkins Motor Stereotypy Research Initiative Fund from the Nesbitt-McMaster Foundation, Klump Family, and Graves Family. This publication was also made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research and the Kennedy Krieger Institute’s IDDRC Grant Number P50 HD103538.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Shannon Dean, Laura Tochen, Farhan Augustine, Shreenath Rajendran, and Syed F. Ali. The first draft of the manuscript was written by Shannon Dean, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Johns Hopkins Investigational Review Board (IRB). Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
One author, Shreenath Rajendran, is now employed by Mitokinin, Inc. This company was not involved in the research for this article and the contents do not necessarily reflect the official views of Mitokinin Inc.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dean, S.L., Tochen, L., Augustine, F. et al. The Role of the Cerebellum in Repetitive Behavior Across Species: Childhood Stereotypies and Deer Mice. Cerebellum 21, 440–451 (2022). https://doi.org/10.1007/s12311-021-01301-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-021-01301-3