Abstract
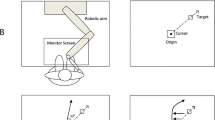
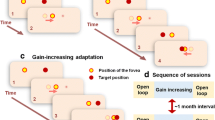
We examined the role of the cerebellum in patients with tremor-dominant cervical dystonia by measuring the adaptive capacity of rapid reflexive eye movements (saccades). We chose the saccade adaptation paradigm because, unlike other motor learning paradigms, the real-time modification of saccades cannot “wait” for the sensory (visual) feedback. Instead, saccades rely primarily on the internal reafference modulated by the cerebellum. The saccade adaptation happens over fast and slow timescales. The fast timescale has poor retention of learned response, while the slow timescale has strong retention. Cerebellar defects resulting in loss of function affect the fast timescale but the slow timescale of saccade adaptation is retained. In contrast, maladaptive cerebellar disorders feature the absence of both fast and slow timescales. We were able to measure both timescales using noninvasive oculography in 6 normal individuals. In contrast, both timescales were absent in 12 patients with tremor-dominant cervical dystonia. These findings are consistent with maladaptive cerebellar outflow as a putative pathophysiological basis for tremor-dominant cervical dystonia.



Similar content being viewed by others
References
Tarsy D, Simon DK. Dystonia. N Engl J Med. 2006;355:818–29.
Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121:547–60.
Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–212.
Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–62.
Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35.
Starr PA, Rau GM, Davis V, Marks WJ Jr, Ostrem JL, Simmons D, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165–76. https://doi.org/10.1152/jn.00971.2004.
Calderon DP, Fremont R, Kraenzlin F, Khodakhah K. The neural substrates of rapid-onset dystonia-parkinsonism. Nat Neurosci. 2011;14:357–65. https://doi.org/10.1038/nn.2753.
Vitek JL, Delong MR, Starr PA, Hariz MI, Metman LV. Intraoperative neurophysiology in DBS for dystonia. Mov Disord. 2011;26(Suppl 1):S31–6. https://doi.org/10.1002/mds.23619.
Lozano AM, Kumar R, Gross RE, Giladi N, Hutchison WD, Dostrovsky JO, et al. Globus pallidus internus pallidotomy for generalized dystonia. Mov Disord. 1997;12:865–70. https://doi.org/10.1002/mds.870120606.
Lenz FA, Suarez JI, Metman LV, Reich SG, Karp BI, Hallett M, et al. Pallidal activity during dystonia: somatosensory reorganisation and changes with severity. J Neurol Neurosurg Psychiatry. 1998;65:767–70.
Neychev V, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–509.
Neychev VK, Gross R, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201.
Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–33.
Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. https://doi.org/10.1016/j.neuroscience.2013.11.062.
Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. https://doi.org/10.1016/j.expneurol.2012.11.019.
Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–90.
Lekhel H, Popov K, Anastasopoulos D, Bronstein A, Bhatia K, Marsden CD, et al. Postural responses to vibration of neck muscles in patients with idiopathic torticollis. Brain. 1997;120(Pt 4):583–91.
Hubsch C, Vidailhet M, Rivaud-Pechoux S, Pouget P, Brochard V, Degos B, et al. Impaired saccadic adaptation in DYT11 dystonia. J Neurol Neurosurg Psychiatry. 2011;82:1103–6. https://doi.org/10.1136/jnnp.2010.232793.
Katschnig-Winter P, Schwingenschuh P, Davare M, Sadnicka A, Schmidt R, Rothwell JC, et al. Motor sequence learning and motor adaptation in primary cervical dystonia. J Clin Neurosci. 2014;21:934–8. https://doi.org/10.1016/j.jocn.2013.08.019.
Sadnicka A, Patani B, Saifee TA, Kassavetis P, Parees I, Korlipara P, et al. Normal motor adaptation in cervical dystonia: a fundamental cerebellar computation is intact. Cerebellum. 2014;13:558–67. https://doi.org/10.1007/s12311-014-0569-0.
Sadnicka A, Stevenson A, Bhatia KP, Rothwell JC, Edwards MJ, Galea JM. High motor variability in DYT1 dystonia is associated with impaired visuomotor adaptation. Sci Rep. 2018;8:3653. https://doi.org/10.1038/s41598-018-21545-0.
LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18:60–9. https://doi.org/10.1002/mds.10301.
Kumandas S, Per H, Gumus H, Tucer B, Yikilmaz A, Kontas O, et al. Torticollis secondary to posterior fossa and cervical spinal cord tumors: report of five cases and literature review. Neurosurg Rev. 2006;29:333–8; discussion 8. https://doi.org/10.1007/s10143-006-0034-8.
Cancel G, Durr A, Didierjean O, Imbert G, Burk K, Lezin A, et al. Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum Mol Genet. 1997;6:709–15. https://doi.org/10.1093/hmg/6.5.709.
Hagenah JM, Zuhlke C, Hellenbroich Y, Heide W, Klein C. Focal dystonia as a presenting sign of spinocerebellar ataxia 17. Mov Disord. 2004;19:217–20. https://doi.org/10.1002/mds.10600.
Lang AE, Rogaeva EA, Tsuda T, Hutterer J, St George-Hyslop P. Homozygous inheritance of the Machado-Joseph disease gene. Ann Neurol. 1994;36:443–7. https://doi.org/10.1002/ana.410360318.
Le Ber I, Clot F, Vercueil L, Camuzat A, Viemont M, Benamar N, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–73. https://doi.org/10.1212/01.wnl.0000244484.60489.50.
Batla A, Sanchez MC, Erro R, Ganos C, Stamelou M, Balint B, et al. The role of cerebellum in patients with late onset cervical/segmental dystonia?--evidence from the clinic. Parkinsonism Relat Disord. 2015;21:1317–22. https://doi.org/10.1016/j.parkreldis.2015.09.013.
DeSimone JC, Archer DB, Vaillancourt DE, Wagle SA. Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain. 2019;142:1644–59. https://doi.org/10.1093/brain/awz085.
Merola A, Dwivedi AK, Shaikh AG, Tareen TK, Da Prat GA, Kauffman MA, et al. Head tremor at disease onset: an ataxic phenotype of cervical dystonia. J Neurol. 2019;266:1844–51. https://doi.org/10.1007/s00415-019-09341-w.
Ghasia FF, Gulati D, Westbrook EL, Shaikh AG. Viewing condition dependence of the gaze-evoked nystagmus in Arnold Chiari type 1 malformation. J Neurol Sci. 2014;339:134–9. https://doi.org/10.1016/j.jns.2014.01.045.
Ghasia FF, Shaikh AG. Uncorrected myopic refractive error increases microsaccade amplitude. Invest Ophthalmol Vis Sci. 2015;56:2531–5. https://doi.org/10.1167/iovs.14-15882.
Ghasia FF, Wilmot G, Ahmed A, Shaikh AG. Strabismus and micro-opsoclonus in Machado-Joseph disease. Cerebellum. 2016;15:491–7. https://doi.org/10.1007/s12311-015-0718-0.
Shaikh AG, Ghasia FF. Novel eye movement disorders in Whipple’s disease-staircase horizontal saccades, gaze-evoked nystagmus, and esotropia. Front Neurol. 2017;8:321. https://doi.org/10.3389/fneur.2017.00321.
Shaikh AG, Ghasia FF. Fixational saccades are more disconjugate in adults than in children. PLoS One. 2017;12:e0175295. https://doi.org/10.1371/journal.pone.0175295.
Shaikh AG, Ghasia FF. Misdirected horizontal saccades in pan-cerebellar atrophy. J Neurol Sci. 2015;355:125–30. https://doi.org/10.1016/j.jns.2015.05.042.
Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Brain Res Cogn Brain Res. 2002;13:41–52. https://doi.org/10.1016/s0926-6410(01)00088-x.
Ethier V, Zee DS, Shadmehr R. Spontaneous recovery of motor memory during saccade adaptation. J Neurophysiol. 2008;99:2577–83. https://doi.org/10.1152/jn.00015.2008.
Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci U S A. 2008;105:7309–14. https://doi.org/10.1073/pnas.0706032105.
Straube A, Deubel H. Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vis Res. 1995;35:3451–8. https://doi.org/10.1016/0042-6989(95)00076-q.
Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci. 2008;28:13929–37. https://doi.org/10.1523/JNEUROSCI.3470-08.2008.
Schnier F, Lappe M. Differences in intersaccadic adaptation transfer between inward and outward adaptation. J Neurophysiol. 2011;106:1399–410. https://doi.org/10.1152/jn.00236.2011.
Kommerell G, Olivier D, Theopold H. Adaptive programming of phasic and tonic components in saccadic eye movements. Investigations of patients with abducens palsy. Investig Ophthalmol. 1976;15:657–60.
Leznik E, Makarenko V, Llinas R. Electrotonically mediated oscillatory patterns in neuronal ensembles: an in vitro voltage-dependent dye-imaging study in the inferior olive. J Neurosci. 2002;22:2804–15.
Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci U S A. 2002;99:449–54. https://doi.org/10.1073/pnas.012604899.
Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci. 2007;10:779–86. https://doi.org/10.1038/nn1901.
Carey MR. Synaptic mechanisms of sensorimotor learning in the cerebellum. Curr Opin Neurobiol. 2011;21:609–15. https://doi.org/10.1016/j.conb.2011.06.011.
Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. https://doi.org/10.1371/journal.pbio.0040179.
Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci. 2009;29:12930–9. https://doi.org/10.1523/JNEUROSCI.3115-09.2009.
Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19:10931–9.
Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008;27:132–44. https://doi.org/10.1111/j.1460-9568.2007.05996.x.
Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83:2047–62.
Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–31.
Panouilleres M, Alahyane N, Urquizar C, Salemme R, Nighoghossian N, Gaymard B, et al. Effects of structural and functional cerebellar lesions on sensorimotor adaptation of saccades. Exp Brain Res. 2013;231:1–11. https://doi.org/10.1007/s00221-013-3662-6.
Panouilleres M, Neggers SF, Gutteling TP, Salemme R, van der Stigchel S, van der Geest JN, et al. Transcranial magnetic stimulation and motor plasticity in human lateral cerebellum: dual effect on saccadic adaptation. Hum Brain Mapp. 2012;33:1512–25. https://doi.org/10.1002/hbm.21301.
Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–84. https://doi.org/10.1152/jn.00822.2009.
Shaikh AG, Hong S, Liao K, Tian J, Solomon D, Zee DS, et al. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain. 2010;133:923–40. https://doi.org/10.1093/brain/awp323.
Shaikh AG, Wong AL, Optican LM, Zee DS. Impaired motor learning in a disorder of the inferior olive: is the cerebellum confused? Cerebellum. 2017;16:158–67. https://doi.org/10.1007/s12311-016-0785-x.
Andreescu CE, Milojkovic BA, Haasdijk ED, Kramer P, De Jong FH, Krust A, et al. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J Neurosci. 2007;27:10832–9. https://doi.org/10.1523/JNEUROSCI.2588-07.2007.
Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–28. https://doi.org/10.1016/j.neuron.2010.07.009.
Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. https://doi.org/10.1016/j.neuron.2011.02.044.
Zoons E, Booij J, Nederveen AJ, Dijk JM, Tijssen MA. Structural, functional and molecular imaging of the brain in primary focal dystonia—a review. Neuroimage. 2011;56:1011–20. https://doi.org/10.1016/j.neuroimage.2011.02.045.
LeDoux MS, Hurst DC, Lorden JF. Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience. 1998;86:533–45. https://doi.org/10.1016/s0306-4522(98)00007-4.
LeDoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res. 2002;145:457–67. https://doi.org/10.1007/s00221-002-1127-4.
LeDoux MS, Lorden JF. Abnormal cerebellar output in the genetically dystonic rat. Adv Neurol. 1998;78:63–78.
LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120:302–10. https://doi.org/10.1006/exnr.1993.1064.
LeDoux MS, Lorden JF, Meinzen-Derr J. Selective elimination of cerebellar output in the genetically dystonic rat. Brain Res. 1995;697:91–103. https://doi.org/10.1016/0006-8993(95)00792-o.
Alvarez-Fischer D, Grundmann M, Lu L, Samans B, Fritsch B, Moller JC, et al. Prolonged generalized dystonia after chronic cerebellar application of kainic acid. Brain Res. 2012;1464:82–8. https://doi.org/10.1016/j.brainres.2012.05.007.
Acknowledgments
This work was supported by American Academy of Neurology Career Award (AS), George C. Cotzias Memorial Fellowship from American Parkinson’s Disease Association (AS), and Dystonia Medical Research Foundation Brain Circuits in Dystonia Research Grant (AS), NIH U54 NS116025-09 (HJ). AM was supported by the Dystonia Medical Research Foundation Clinical Fellowship. PG was supported by the philanthropic support to the University Hospitals Cleveland Medical Center—The Alan Woll Fund.
Funding
Dr. Shaikh is a section editor of The Cerebellum and Guest Editor of The Journal of Computational Neuroscience. Dr. Shaikh has received speaker honoraria from Acorda Therapeutics and editorial honoraria from Elsevier. Dr. Shaikh has received grant support from The American Academy of Neurology, American Parkinson’s Disease Association, and Dystonia Medical Research Foundation. Dr. Espay has received grant support from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, Acadia, Acorda, Cynapsus/Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, the American Academy of Neurology, and the Movement Disorders Society. He serves as Associate Editor of the Journal of Clinical Movement Disorders and on the editorial boards of the Journal of Parkinson’s Disease and Parkinsonism and Related Disorders. H. A. Jinnah has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now), academically oriented institutions (the Dystonia Study Group), and industry (Cavion Therapeutics, Ipsen Pharmaceuticals, Retrophin Inc.). Dr. Jinnah has also served on advisory boards or as a consultant for Abide Therapeutics, Allergan Inc., CoA Therapeutics, Medtronic Inc., Psyadon Pharmaceuticals, Retrophin Inc., and Saol Therapeutics. He has received honoraria or stipends for lectures or administrative work from the American Academy of Neurology, the American Neurological Association, the Dystonia Medical Research Foundation, the International Neurotoxin Society, the International Parkinson’s Disease and Movement Disorders Society, The Parkinson’s Disease Foundation, Tyler’s Hope for a Cure. Dr. Jinnah serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, The Tourette Association of American, and Tyler’s Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which has received the majority of its support through NIH grants NS065701 and NS116025 from the National Institutes of Neurological Disorders and previously from TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences. The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, Beat Dystonia, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Europe, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The University of Cincinnati Institutional review board approved the study protocol and informed consent form that followed the Declaration of Helsinki.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahajan, A., Gupta, P., Jacobs, J. et al. Impaired Saccade Adaptation in Tremor-Dominant Cervical Dystonia—Evidence for Maladaptive Cerebellum. Cerebellum 20, 678–686 (2021). https://doi.org/10.1007/s12311-020-01104-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01104-y