Abstract
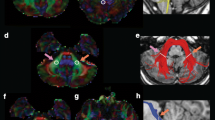
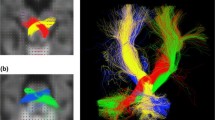
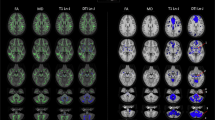
Cerebellum-cerebrum connections are essential for many motor and cognitive functions and cerebellar disorders are prevalent in childhood. The middle (MCP), inferior (ICP), and superior cerebellar peduncles (SCP) are the major white matter pathways that permit communication between the cerebellum and the cerebrum. Knowledge about the microstructural properties of these cerebellar peduncles across childhood is limited. Here, we report on a diffusion magnetic resonance imaging tractography study to describe age-dependent characteristics of the cerebellar peduncles in a cross-sectional sample of infants, children, and adolescents from newborn to 17 years of age (N = 113). Scans were collected as part of clinical care; participants were restricted to those whose scans showed no abnormal findings and whose history and exam had no risk factors for cerebellar abnormalities. A novel automated tractography protocol was applied. Results showed that mean tract-FA increased, while mean tract-MD decreased from infancy to adolescence in all peduncles. Rapid changes were observed in both diffusion measures in the first 24 months of life, followed by gradual change at older ages. The shape of the tract profiles was similar across ages for all peduncles. These data are the first to characterize the variability of diffusion properties both across and within cerebellar white matter pathways that occur from birth through later adolescence. The data represent a rich normative data set against which white matter alterations seen in children with posterior fossa conditions can be compared. Ultimately, the data will facilitate the identification of sensitive biomarkers of cerebellar abnormalities.







Similar content being viewed by others
References
Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87.
Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20(3):236–60.
Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–65.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34.
Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children. Brain. 2000;123(5):1041–50.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–79.
Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130(10):2646–60.
Arora RS, Alston RD, Eden TOB, Estlin EJ, Moran A, Birch JM. Age–incidence patterns of primary CNS tumors in children, adolescents. and adults in England Neuro Oncol. 2009;11(4):403–13.
Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–95.
Messerschmidt A, Prayer D, Brugger PC, Boltshauser E, Zoder G, Sterniste W, et al. Preterm birth and disruptive cerebellar development: assessment of perinatal risk factors. Eur J Paediatr Neurol. 2008;12(6):455–60.
Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–24.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11(3):777–807.
Naidich TP, Duvernoy HM. Duvernoy’s atlas of the human brain stem and cerebellum: high-field MRI : surface anatomy, internal structure, vascularization and 3D sectional anatomy. Springer; 2009. 876 p.
Stieltjes B, Kaufmann WE, van Zijl PCM, Fredericksen K, Pearlson GD, Solaiyappan M, et al. Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage. 2001;14(3):723–35.
Messerschmidt A, Brugger PC, Boltshauser E, Zoder G, Sterniste W, Birnbacher R, et al. Disruption of cerebellar development: potential complication of extreme prematurity. Am J Neuroradiol. 2005;26(7):1659–67.
Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, et al. Long-term outcome of brain structure in premature infants. Arch Pediatr Adolesc Med. 2011;165(5):443–50.
Limperopoulos C. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116(4):844–50.
Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46.
Deoni SCL, Dean DC, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63(3):1038–53.
Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005 Dec;15(12):1848–54.
Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–58.
Saksena S, Husain N, Malik GK, Trivedi R, Sarma M, Rathore RS, et al. Comparative evaluation of the cerebral and cerebellar white matter development in pediatric age group using quantitative diffusion tensor imaging. Cerebellum. 2008;7(3):392–400.
Leitner Y, Travis KE, Ben-Shachar M, Yeom KW, Feldman HM. Tract profiles of the cerebellar white matter pathways in children and adolescents. Cerebellum. 2015;14(6):613–23.
Travis KE, Leitner Y, Feldman HM, Ben-Shachar M. Cerebellar white matter pathways are associated with reading skills in children and adolescents. Hum Brain Mapp. 2015;36(4):1536–53.
Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11).
Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36(4):1292–313.
Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71(10):881–9.
Rohde GK, Barnett AS, Basser PJ, Marenco S, Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51(1):103–14.
Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, Ito H, et al. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am J Neuroradiol. 2007;28(6):1102–6.
Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med. 2009;61(6):1336–49.
Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–9.
Rose J, Vassar R, Cahill-Rowley K, Guzman XS, Stevenson DK, Barnea-Goraly N. Brain microstructural development at near-term age in very-low-birth-weight preterm infants: an atlas-based diffusion imaging study. Neuroimage. 2014;86:244–56.
de Bruïne FT, van Wezel-Meijler G, Leijser LM, van den Berg-Huysmans AA, van Steenis A, van Buchem MA, et al. Tractography of developing white matter of the internal capsule and corpus callusom in very preterm infants. Eur Radiol. 2011;21(3):538–47.
Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. Neuroimage. 2004;23(1):21–4.
Dodson CK, Travis KE, Ben-Shachar M, Feldman HM. White matter microstructure of 6-year old children born preterm and full term. NeuroImage Clin. 2017;16:268–75.
Fingher N, Dinstein I, Ben-Shachar M, Haar S, Dale AM, Eyler L, et al. Toddlers later diagnosed with autism exhibit multiple structural abnormalities in temporal corpus callosum fibers. Cortex. 2017;97:291–305.
Schneider JFL, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–66.
Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30(4):1121–32.
Partridge SC, Mukherjee P, Berman JI, Henry RG, Miller SP, Lu Y, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22(4):467–74.
Yeh T-H, Lee DY, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia. 2009;57(11):1239–49.
Vasung L, Huang H, Jovanov-Milošević N, Pletikos M, Mori S, Kostović I. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat. 2010;217(4):400–17.
Yakovlev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. Regional development of the brain in early life. 1967.
Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47(3):217–34.
Mezer A, Yeatman JD, Stikov N, Kay KN, Cho N, Dougherty RF, et al. Quantifying the local tissue volume and composition in individual brains with MRI HHS Public Access. Nat Med. 2013;19(12):1667–72.
Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. Axcaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59(6):1347–54.
Tournier J-D, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage. 2008;42(2):617–25.
Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–82.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–16.
Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15(7–8):435–55.
Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC. Changes in brain water diffusion during childhood. Neuroradiology. 1999;41(12):929–34.
Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, et al. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49(1):94–103.
Hermoye L, Saint-Martin C, Cosnard G, Lee S-K, Kim J, Nassogne M-C, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29(2):493–504.
Doshi J, Erus G, Ou Y, Resnick SM, Gur RC, Gur RE, et al. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186–95.
Iglesias JE, Sabuncu MR. Multi-atlas segmentation of biomedical images: a survey. Med Image Anal. 2015;24(1):205–19.
Funding
This study was funded by the following grants: Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (RO1 HD069162 to Feldman, PI and 5K99HD084749 to Travis, PI) and the Stanford Transdisciplinary Initiatives Program, Child Health Research Institute (1186741-100-DHDHY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
(DOCX 188 kb)
Online Resource 2
(DOCX 16.6 kb)
Online Resource 3
(DOCX 15.8 kb)
Online Resource 4
(DOCX 17.5 kb)
Online Resource 5
(DOCX 17.6 kb)
Rights and permissions
About this article
Cite this article
Bruckert, L., Shpanskaya, K., McKenna, E.S. et al. Age-Dependent White Matter Characteristics of the Cerebellar Peduncles from Infancy Through Adolescence. Cerebellum 18, 372–387 (2019). https://doi.org/10.1007/s12311-018-1003-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-1003-9