Abstract
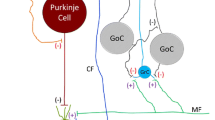
Ethanol exposures during the early postnatal period of the rat result in significant death of Purkinje cells (PCs). The magnitude, time-course, and lobular specificity of PC death have been well characterized in several studies. Additionally, significant reduction of climbing fiber inputs to the surviving PCs has been characterized. This study investigates whether further alterations to the cerebellar cortical circuits might occur as a result of developmental ethanol exposures. We first examined the firing pattern of PCs in acute slice preparations on postnatal days 13–15. While the basic firing frequency was not significantly altered, PCs from rat pups treated with ethanol on postnatal days 4–6 showed a significantly increased number of inhibitory postsynaptic potentials (IPSCs) and a larger Ih current. We conducted immunofluorescent studies to identify the probable cause of the increased IPSCs. We found a significant 21 % increase in the number of basket cells per PC and a near doubling of the volume of co-localized basket cell axonal membrane with PC. In addition, we identified a significant (~147 %) increase in HCN1 channel volume co-localized to PC volume. Therefore, the cerebellar cortex that survives targeted postnatal ethanol exposure is dramatically altered in development subsequent to PC death. The cerebellar cortical circuit that results is one that operates under a significant degree of increased resting inhibition. The alterations in the development of cerebellar circuitry following ethanol exposure, and the significant loss of PCs, could result in modifications of the structure and function of other brain regions that receive cerebellar inputs.







Similar content being viewed by others
References
Office of Applied Studies, Substance use among women during pregnancy and following childbirth, in The NSDUH Report. 2009, Substance Abuse and Mental Health Services Administration: Rockville.
Sidhu S, Floyd R. Alcohol use among women of childbearing age—United States, 1991-1999. MMWR Morb Mortal Wkly Rep. 2002;51(13):273–6.
Popova S, Chambers C. Fetal alcohol spectrum disorders must be recognized globally as a large public health problem. Int J Alcohol Drug Res. 2014;3(1):3.
Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21(2):73–80.
Jones KL. The effects of alcohol on fetal development. Birth Defects Res C Embryo Today. 2011;93(1):3–11.
Dorrie, N., et al. Fetal alcohol spectrum disorders. Eur Child Adolesc Psychiatry. 2014;23(10):863–75.
Lebel C et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32(44):15243–51.
Norman AL et al. A functional magnetic resonance imaging study of spatial working memory in children with prenatal alcohol exposure: contribution of familial history of alcohol use disorders. Alcohol Clin Exp Res. 2013;37(1):132–40.
Connor PD et al. Effects of prenatal alcohol exposure on fine motor coordination and balance: a study of two adult samples. Neuropsychologia. 2006;44(5):744–51.
Mattson SN et al. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–53.
Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):339–44.
Sowell ER et al. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res. 1996;20(1):31–4.
Autti-Ramo I et al. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44(2):98–106.
Archibald SL et al. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–54.
Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 2011;21(2):133–47.
du Plessis, L., et al. An in vivo 1H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2014;38(5):1330–8.
Chen X et al. Understanding specific effects of prenatal alcohol exposure on brain structure in young adults. Hum Brain Mapp. 2012;33(7):1663–76.
Roebuck TM et al. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998;22(1):252–8.
Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3(3):178–87.
Coffin JM et al. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41(3):389–98.
Jacobson SW et al. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–72.
Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood). 2005;230(6):389–93.
Dobbing J, Sands J. The quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–67.
Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83.
Ethen MK et al. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13(2):274–85.
Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14(1):107–18.
Dikranian K et al. Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Brain Res Dev Brain Res. 2005;155(1):1–13.
Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces brain weight restriction and cerebellar Purkinje cell loss. Alcohol. 1990;7(2):107–14.
Goodlett CR et al. Binge-like alcohol exposure of neonatal rats via intragastric intubation induces both Purkinje cell loss and cortical astrogliosis. Alcohol Clin Exp Res. 1997;21(6):1010–7.
Pierce DR, Williams DK, Light KE. Purkinje cell vulnerability to developmental ethanol exposure in the rat cerebellum. Alcohol Clin Exp Res. 1999;23(10):1650–9.
Altman, J. Morphological development of the rat cerebellum and some of its mechanisms. Exp Brain Res (Suppl). 1982;6:8–49.
Altman, J. and S.A. Bayer, Development of the cerebellar system: in relation to its evolution, structure, and functions. Boca Raton, CRC Press; 1996.
Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17(3):610–22.
Pierce DR et al. Developmental alterations in olivary climbing fiber distribution following postnatal ethanol exposure in the rat. Neuroscience. 2010;169:1438–48.
Idrus NM, Napper RMA. Acute and long-term Purkinje cell loss following a single Ethanol binge during the early third trimester equivalent in the rat. Alcohol Clin Exp Res. 2012;36(8):1365–73.
Goodlett, C.R. and K.R. Lundahl. Age-related changes in vulnerability to alcohol-induced Purkinje cell loss in the first neonatal week in rats. Society for Neuroscience Abstracts. 2001;27(2):2334.
Light KE, Belcher SR, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002;114(2):327–37.
Tran TD et al. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res. 2005;29(1):117–29.
Luo J. Mechanisms of ethanol-induced death of cerebellar granule cells. Cerebellum. 2012;11(1):145–54.
Bauer-Moffett C, Altman J. Ethanol-induced reductions in cerebellar growth of infant rats. Exp Neurol. 1975;48:378–82.
Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: a morphological study. Brain Res. 1977;119(2):249–68.
Marcussen BL et al. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol. 1994;11(2):147–56.
Napper RM, West JR. Permanent neuronal cell loss in the cerebellum of rats exposed to continuous low blood alcohol levels during the brain growth spurt: a stereological investigation. J Comp Neurol. 1995;362(2):283–92.
Napper RM, West JR. Permanent neuronal cell loss in the inferior olive of adult rats exposed to alcohol during the brain growth spurt: a stereological investigation. Alcohol Clin Exp Res. 1995;19(5):1321–6.
Hioki H et al. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience. 2003;117(1):1–6.
Miyazaki T et al. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci. 2003;17(12):2563–72.
Takagishi Y et al. Diminished climbing fiber innervation of Purkinje cells in the cerebellum of myosin Va mutant mice and rats. Dev Neurobiol. 2007;67(7):909–23.
Pierce DR et al. Olivary climbing fiber alterations in PN 40 rat cerebellum following postnatal ethanol exposure. Brain Res. 2011;1378:54–65.
Backman C et al. Electrophysiological characterization of cerebellar neurons from adult rats exposed to ethanol during development. Alcohol Clin Exp Res. 1998;22(5):1137–45.
Zamudio-Bulcock, P.A., R.A. Morton, and C.F. Valenzuela. Third trimester-equivalent ethanol exposure does not alter complex spikes and climbing fiber long-term depression in cerebellar Purkinje neurons from juvenile rats. Alcohol Clin Exp Res. 2014;38(5):1293–1300.
Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48(1):19–47.
Light KE et al. Intragastric intubation: important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcohol Clin Exp Res. 1998;22(7):1600–6.
Alcami P et al. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32(9):3118–30.
Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci. 2005;25(36):8197–208.
Hayar A et al. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004;24(5):1190–9.
Heck DH, Thach WT, Keating JG. On-beam synchrony in the cerebellum as the mechanism for the timing and coordination of movement. Proc Natl Acad Sci U S A. 2007;104(18):7658–63.
Kosaka T et al. Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Exp Brain Res. 1993;93(3):483–91.
Wadleigh A, Valenzuela CF. Ethanol increases GABAergic transmission and excitability in cerebellar molecular layer interneurons from GAD67-GFP knock-in mice. Alcohol Alcohol. 2012;47(1):1–8.
Jeong YG, Hyun BH, Hawkes R. Abnormalities in cerebellar Purkinje cells in the novel ataxic mutant mouse, pogo. Brain Res Dev Brain Res. 2000;125(1–2):61–7.
Shin M, Chetkovich DM. Activity-dependent regulation of h channel distribution in hippocampal CA1 pyramidal neurons. J Biol Chem. 2007;282(45):33168–80.
McKay BE, Turner RW. Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol. 2005;567(Pt 3):829–50.
Watt AJ et al. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;12(4):463–73.
Pierce DR, Goodlett CR, West JR. Differential neuronal loss following early postnatal alcohol exposure. Teratology. 1989;40:113–26.
Pierce DR, Serbus DC, Light KE. Intragastric intubation of alcohol during the postnatal development of rats results in selective cell loss in the cerebellum. Alcohol Clin Exp Res. 1993;17(6):1275–80.
Hayar A, Ennis M. Endogenous GABA and glutamate finely tune the bursting of olfactory bulb external tufted cells. J Neurophysiol. 2007;98(2):1052–6.
Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19(3):665–78.
de Solages C et al. High-frequency organization and synchrony of activity in the Purkinje cell layer of the cerebellum. Neuron. 2008;58(5):775–88.
Shin SL, De Schutter E. Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol. 2006;96(6):3485–91.
Yan H et al. Developmental sensitivity of hippocampal interneurons to ethanol: involvement of the hyperpolarization-activated current. Ih J Neurophysiol. 2009;101(1):67–83.
Ming Z et al. Competing presynaptic and postsynaptic effects of ethanol on cerebellar Purkinje neurons. Alcohol Clin Exp Res. 2006;30(8):1400–7.
Cameron DB et al. Four distinct phases of basket/stellate cell migration after entering their final destination (the molecular layer) in the developing cerebellum. Dev Biol. 2009;332(2):309–24.
Ichikawa R et al. Developmental switching of perisomatic innervation from climbing fibers to basket cell fibers in cerebellar Purkinje cells. J Neurosci. 2011;31(47):16916–27.
Sotelo C. Development of “Pinceaux” formations and dendritic translocation of climbing fibers during the acquisition of the balance between glutamatergic and gamma-aminobutyric acidergic inputs in developing Purkinje cells. J Comp Neurol. 2008;506(2):240–62.
Lujan R et al. Preferential localization of the hyperpolarization-activated cyclic nucleotide-gated cation channel subunit HCN1 in basket cell terminals of the rat cerebellum. Eur J Neurosci. 2005;21(8):2073–82.
Oldfield CS, Marty A, Stell BM. Interneurons of the cerebellar cortex toggle Purkinje cells between up and down states. Proc Natl Acad Sci U S A. 2010;107(29):13153–8.
Aitken PG et al. Preparative methods for brain slices: a discussion. J Neurosci Methods. 1995;59(1):139–49.
Ting JT et al. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol. 2014;1183:221–42.
Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–8.
Vincent P, Marty A. Neighboring cerebellar Purkinje cells communicate via retrograde inhibition of common presynaptic interneurons. Neuron. 1993;11(5):885–93.
D'Angelo E et al. Synaptic excitation of individual rat cerebellar granule cells in situ: evidence for the role of NMDA receptors. J Physiol. 1995;484(Pt 2):397–413.
Rieubland S, Roth A, Hausser M. Structured connectivity in cerebellar inhibitory networks. Neuron. 2014;81(4):913–29.
Lin XB et al. The fine temporal structure of the rat licking pattern: what causes the variabiliy in the interlick intervals and how is it affected by the drinking solution? Chem Senses. 2013;38(8):685–704.
Rinaldi A et al. HCN1 channels in cerebellar Purkinje cells promote late stages of learning and constrain synaptic inhibition. J Physiol. 2013;591(Pt 22):5691–709.
Kim CH et al. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol. 2012;590(Pt 2):273–88.
Zhou H et al. Cerebellar modules operate at different frequencies. Elife. 2014;3:e02536.
Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol. 2008;100(6):3417–28.
Vincent P, Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol. 1996;494(Pt 1):183–99.
Sugihara I, Lang EJ, Llinas R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–71.
Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32(6):1044–55.
Middleton SJ et al. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58(5):763–74.
Traub RD et al. Model of very fast (>75 Hz) network oscillations generated by electrical coupling between the proximal axons of cerebellar Purkinje cells. Eur J Neurosci. 2008;28(8):1603–16.
Angelo K et al. Local and global effects of I(h) distribution in dendrites of mammalian neurons. J Neurosci. 2007;27(32):8643–53.
Chang W, Strahlendorf JC, Strahlendorf HK. Ionic contributions to the oscillatory firing activity of rat Purkinje cells in vitro. Brain Res. 1993;614(1–2):335–41.
Crepel F, Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986;372:1–23.
Nolan MF et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115(5):551–64.
Williams SR et al. Membrane potential bistability is controlled by the hyperpolarization-activated current I(H) in rat cerebellar Purkinje neurons in vitro. J Physiol. 2002;539(Pt 2):469–83.
Wise AK et al. Mechanisms of synchronous activity in cerebellar Purkinje cells. J Physiol. 2010;588(Pt 13):2373–90.
Rogers TD et al. Connecting the dots of the cerebro-cerebellar role in cognitive function: Neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse. 2011;65(11):1204–12.
Stoodley C. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–65.
Wang SS-H, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518–32.
Courchesne E et al. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318(21):1349–54.
Riva D et al. Gray matter reduction in the vermis and CRUS-II is associated with social and Interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL study. Cerebellum. 2013;12(5):676–85.
Reeb-Sutherland, B.C. and N.A. Fox, Eyeblink conditioning: a non-invasive biomarker for neurodevelopmental disorders. J Autism Dev Disord, 2013;45(2):376–94.
Wagner JL et al. Rehabilitation training using complex motor learning rescues deficits in eyeblink classical conditioning in female rats induced by binge-like neonatal alcohol exposure. Alcohol Clin Exp Res. 2013;37(9):1561–70.
Acknowledgments
This work was supported in part by the Center of Excellence Award P30 GM110702. The authors thank the University of Central Arkansas for the use of their Zeiss Pascal confocal microscope.
Conflicts of Interest
The authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Light, K.E., Hayar, A.M. & Pierce, D.R. Electrophysiological and Immunohistochemical Evidence for an Increase in GABAergic Inputs and HCN Channels in Purkinje Cells that Survive Developmental Ethanol Exposure. Cerebellum 14, 398–412 (2015). https://doi.org/10.1007/s12311-015-0651-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0651-2