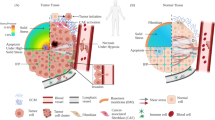
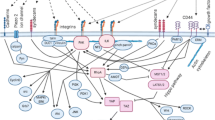
Abstract
Though the existence of cancer stem cells remained enigmatic initially, over the time their participation in tumorigenesis and tumor progression has become highly evident. Today, they are also appreciated as the causal element for tumor heterogeneity and drug-resistance. Cancer stem cells activate a set of molecular pathways some of which are triggered by the unique mechanical properties of the tumor tissue stroma. A relatively new field called mechanobiology has emerged, which aims to critically evaluate the mechanical properties associated with biological events like tissue morphogenesis, cell-cell or cell-matrix interactions, cellular migration and also the development and progression of cancer. Development of more realistic model systems and biophysical instrumentation for observation and manipulation of cell-dynamics in real-time has invoked a hope for some novel therapeutic modalities against cancer in the future. This review discusses the fundamental concepts of cancer stem cells from an intriguing viewpoint of mechanobiology and some important breakthroughs to date.



Similar content being viewed by others
Abbreviations
- CSC:
-
Cancer stem cell
- EMT:
-
Epithelial-mesenchymal transition
References
Zhang J, Li L (2008) Stem cell niche: microenvironment and beyond. J Biol Chem 283(15):9499–9503. https://doi.org/10.1074/jbc.R700043200
Clarke MF, Dick JE, Dirks PB et al (2006) Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res 66:9339–9344. https://doi.org/10.1158/0008-5472.CAN-06-3126
Plaks V, Kong N, Werb Z (2015) The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16(3):225–238. https://doi.org/10.1016/j.stem.2015.02.015
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 3:891–906. https://doi.org/10.1016/j.cell.2009.10.027
Paszek MJ, Zahir N, Johnson KR et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254. https://doi.org/10.1016/j.ccr.2005.08.010
Boyd NF, Rommens JM, Vogt K et al (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6(10):798–808. https://doi.org/10.1016/S1470-2045(05)70390-9
Daniels CE, Jett JR (2005) Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med 11(5):431–437. https://doi.org/10.1097/01.mcp.0000170521.71497.ba
Broders-Bondon F, Ho-Bouldoires THN, Fernandez-Sanchez ME, Farge E (2018) Mechanotransduction in tumor progression: the dark side of the force. J Cell Biol 217(5):1571–1587. https://doi.org/10.1083/jcb.201701039
Jacobs CR, Hayden H, Kwon RY (2012) Cell Mechanics in the laboratory. In: Scholl S (ed) Introduction to cell mechanics and mechanobiology. 1st edn. Garland Science, New York and London, pp 151–179
McLane JS, Ligon LA (2016) Stiffened extracellular matrix and signaling from stromal fibroblasts via osteoprotegerin regulate tumor cell Invasionin a 3-D tumor in situ model. Cancer Microenviron 9:127–139. https://doi.org/10.1007/s12307-016-0188-z
Xu Z, Li E, Guo Z et al (2016) Design and construction of a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis. ACS Appl Mater Interfaces 8(39):25840–25847. https://doi.org/10.1021/acsami.6b08746
Duinen VV, Trietsch SJ, Joore J, Vulto P, Hankemeier T (2015) Microfluidic 3D cell culture: from tools to tissue models. Curr Opin Biotechnol 35:118–126. https://doi.org/10.1016/j.copbio.2015.05.002
Bonnet D, Dick J (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737. https://doi.org/10.1038/nm0797-730
Schepers AG, Snippert HJ, Stange DE et al (2012) Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337:730–735. https://doi.org/10.1126/science.1224676
Al-Hajj M, Wicha M, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100:3983–3988. https://doi.org/10.1073/pnas.0530291100
Singh S, Hawkins C, Clarke I, Squire J (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. https://doi.org/10.1038/nature03128
Luo Y, Dallaglio K, Chen Y et al (2012) Aldh1a isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells 30:2100–2113. https://doi.org/10.1002/stem.1193
Shackleton M, Quintana E, Fearon ER, Morrison SJ (2009) Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138:822–829. https://doi.org/10.1016/j.cell.2009.08.017
Li Y, Kong D, Ahmad A et al (2012) Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett 338(1):94–100. https://doi.org/10.1016/j.canlet.2012.03.018
Huntly BJ, Gilliland DG (2005) Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 5:311–321. https://doi.org/10.1038/nrc1592
Tirino V, Desiderio V, Paino F et al (2012) Methods for cancer stem cell detection and isolation. Methods Mol Biol 879:513–529. https://doi.org/10.1007/978-1-61779-815-3_32
Zhang Y, Wu M, Han X et al (2015) High-throughput, label-free isolation of cancer stem cells on the basis of cell adhesion capacity. Angew Chem Int Ed Engl 54(37):10838–10842. https://doi.org/10.1002/anie.201505294
Babahosseini H, Ketene AN, Schmelz EM et al (2014) Biomechanical profile of cancer stem-like/tumor initiating cells derived from a progressive ovarian cancer model. Nanomedicine 10(5):1013–1019. https://doi.org/10.1016/j.nano.2013.12.009
Mohammadalipour A, Burdick MM, Tees DFJ (2018) Deformability of breast cancer cells in correlation with surface markers and cell rolling. FASEB J 32(4):1806–1817. https://doi.org/10.1096/fj.201700762R
Sun J, Luo Q, Liu L et al (2016) Biomechanical profile of cancer stem like cells derived from MHCC97H cell lines. J Biomech 49(1):45–52. https://doi.org/10.1016/j.jbiomech.2015.11.007
Aponte PM, Caicedo A (2017) Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int 2017:5619472. https://doi.org/10.1155/2017/5619472
Cabrera MC, Hollingsworth RE, Hurt EM (2015) Cancer stem cell plasticity and tumor hierarchy. World J Stem Cells 7(1):27–36. https://doi.org/10.4252/wjsc.v7.i1.27
Chaffer CL, Brueckmann I, Scheel C et al (2011) Normal and neoplastic nonstem cells can spontaneously convert to a stemlike state. Proc Natl Acad Sci U S A 108(19):7950–7955. https://doi.org/10.1073/pnas.1102454108
Borovski T, Melo FSE, Vermeulen L, Medema JP (2011) Cancer stem cell niche: the place to be. Cancer Res 71(3):634–639. https://doi.org/10.1158/0008-5472.CAN-10-3220
Alison MR, Lim S, Nicholoson L (2010) Cancer stem cells: problems for therapy? J Pathol 223:147–161. https://doi.org/10.1002/path.2793
Pang M-F, Siedlik MJ, Han S et al (2016) Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res 76:5277–5287. https://doi.org/10.1158/0008-5472.CAN-16-0579
Matsui WH (2016) Cancer stem cell signaling pathways. Medicine 95(1):S8–S19. https://doi.org/10.1097/MD.0000000000004765
Varelas X, Miller BW, Sopko R et al (2010) The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell 18(4):579–591. https://doi.org/10.1016/j.devcel.2010.03.007
Varelas X, Sakuma R, Samavarchi-Tehrani P et al (2008) TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10(7):837–848. https://doi.org/10.1038/ncb1748
McMurray RJ, Dalby MJ, Tsimbouri PM (2015) Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J Tissue Eng Regen Med 9(5):528–539. https://doi.org/10.1002/term.1957
Faulk DM, Johnson SA, Zhang L, Badylak SF (2014) Role of the extracellular matrix in whole organ engineering. J Cell Physiol 229(8):984–989. https://doi.org/10.1002/jcp.24532
Paget S (1989) The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev 8(2):98–101. https://doi.org/10.1016/S0140-6736(00)49915-0
Huang S, Ingber DE (2005) Cell tension, matrix mechanics, and cancer development. Cancer Cell 8:175–176. https://doi.org/10.1016/j.ccr.2005.08.009
Wang MN, Zhao JZ, Zhang LS et al (2017) Role of tumor microenvironment in tumorigenesis. J Cancer 8:761–773. https://doi.org/10.7150/jca.17648
Correas JM, Tissier AM, Khairoune A et al. (2015) Prostate cancer: diagnostic performance of real-time shear-wave elastography. Radiology 275(1):280–289. https://doi.org/10.1148/radiol.14140567
Boyd NF, Li Q, Melnichouk O et al (2014) Evidence that breast tissue stiffness is associated with risk of breast cancer. PLoS One 9(7):e100937. https://doi.org/10.1371/journal.pone.0100937
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and diseases. Nat Rev Mol Cell Biol 15(12):786–801. https://doi.org/10.1038/nrm3904
Dasari S, Fang Y, Mitra AK (2018) Cancer associated fibroblasts: naughty neighbors that drive ovarian Cancer progression. Cancers 10(11):406. https://doi.org/10.3390/cancers10110406
Sriram G, Bigliardi PL, Bigliardi-Qi M (2015) Fibroblast heterogeneity and its implications for engineering organotypic skin models in vitro. Eur J Cell Biol 94:483–512. https://doi.org/10.1016/j.ejcb.2015.08.001
Li B, Wang JH (2011) Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viability 20:108–120. https://doi.org/10.1016/j.jtv.2009.11.004
Cirri P, Chiarugi P (2011) Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res 1(4):482–497
Chiarugi P (2013) Cancer-associated fibroblasts and macrophages: friendly conspirators for malignancy. OncoImmunology 2(9):e25563. https://doi.org/10.4161/onci.25563
Ronca R, Van Ginderachter J, Turtoi A (2018) Paracrine interactions of cancer-associated fibroblasts, macrophages and endothelial cells. Curr Opin Oncol 30(1):45–53. https://doi.org/10.1097/CCO.0000000000000420
De VK, Rao L, De BE et al (2014) Cancer associated fibroblasts and tumor growth: focus on multiple myeloma. Cancers (Basel) 6(3):1363–1381. https://doi.org/10.3390/cancers6031363
Yeldag G, Rice A, Hernández ADR (2018) Chemoresistance and the self-maintaining tumor microenvironment. Cancers (Basel) 10(12):471. https://doi.org/10.3390/cancers10120471
Zanotelli MR, Reinhart-King CA (2018) Mechanical forces in tumor angiogenesis. Adv Exp Med Biol 1092:91–112. https://doi.org/10.1007/978-3-319-95294-9_6
Hynes RO, Naba A (2011) Overview of the Matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4(1):a004903–a004903. https://doi.org/10.1101/cshperspect.a004903
Yue B (2014) Biology of the extracellular matrix: an overview. J Glaucoma 23(8):S20–S23. https://doi.org/10.1097/IJG.0000000000000108
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406. https://doi.org/10.1083/jcb.201102147
Walker C, Mojares E, del Río Hernández A (2018) Role of extracellular matrix in development and cancer progression. Int J Mol Sci 19(10):3028. https://doi.org/10.3390/ijms19103028
Cox TR, Erler JT (2011) Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 4(2):165–178. https://doi.org/10.1242/dmm.004077
Kalli M, Stylianopoulos T (2018) Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front Oncol 8:55. https://doi.org/10.3389/fonc.2018.00055
Leung DY, Glagov S, Mathews MB (1976) Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191:475–477
Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15(12):802–812. https://doi.org/10.1038/nrm3896
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370. https://doi.org/10.1038/nm.2537
Ferretti S, Allegrini PR, Becquet MM, McSheehy PMJ (2009) Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia 11:874–881. https://doi.org/10.1593/neo.09554
Ng CP, Hinz B, Swartz MA (2005) Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118:4731–4739. https://doi.org/10.1242/jcs.02605
Bao B, Azmi AS, Ali S et al (2012) The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta 1826:272–296. https://doi.org/10.1016/j.bbcan.2012.04.008
Gilkes DM, Semenza GL, Wirtz D (2014) Hypoxia and the extracellular matrix: drivers of tumor metastasis. Nat Rev Cancer 14:430–439. https://doi.org/10.1038/nrc3726
Luis Alonso J, Goldmann WH (2016) Cellular mechanotransduction. AIMS Biophysics 3(1):50–62. https://doi.org/10.3934/biophy.2016.1.50
Jaalouk DE, Lammerding J (2009) Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10:63–73. https://doi.org/10.1038/nrm2597
Wang L, Zuo X, Xie K, Wei D (2017) The role of CD44 and cancer stem cells. Cancer Stem Cells 1692:31–42. https://doi.org/10.1007/978-1-4939-7401-6_3
Kamble SC, Bapat SA (2013) Stem cell and cancer stem cell games on the ECM field. J Cancer Stem Cell Res 1:e1002. https://doi.org/10.14343/JCSCR.2013.1e1002
Glumac PM, LeBeau AM (2018) The role of CD133 in cancer: a concise review. Clin Transl Med 7:18. https://doi.org/10.1186/s40169-018-0198-1
Motegi H, Kamoshima Y, Terasaka S et al (2014) Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology 34:378–385. https://doi.org/10.1111/neup.12117
Song WS, Yang YP, Huang CS et al (2016) Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc 79(10):538–545. https://doi.org/10.1016/j.jcma.2016.03.010
Toy KA, Valiathan RR, Nunez F, Kidwell KM et al (2015) Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat 150:9–18. https://doi.org/10.1007/s10549-015-3285-7
Shimada K, Anai S, Fujii T et al (2013) Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. J Pathol 231(4):495–504. https://doi.org/10.1002/path.4271
Lathia JD, Li M, Hall PE et al (2012) Laminin alpha 2 enables glioblastoma stem cell growth. Ann Neurol 72:766–778. https://doi.org/10.1002/ana.23674
Chang C, Goel HL, Gao H et al (2015) A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes Dev 29:1–6. https://doi.org/10.1101/gad.253682.114
Keire PA, Kang I, Wight TN (2017) Versican: role in cancer tumorigenesis. In: Brekken R, Stupack D (eds) Extracellular matrix in tumor biology. Biology of Extracellular Matrix. Springer, Cham, pp 51–74
Yu Q, Xue Y, Liu J, Xi Z, Li Z, Liu Y (2018) Fibronectin promotes the malignancy of glioma stem-like cells via modulation of cell adhesion, differentiation, proliferation and chemoresistance. Front Mol Neurosci 11:130. https://doi.org/10.3389/fnmol.2018.00130
Stutchbury B, Atherton P, Tsang R et al (2017) Distinct focal adhesion protein modules control different aspects of mechanotransduction. J Cell Sci 130:1612–1624. https://doi.org/10.1242/jcs.195362
Begum A, Ewachiw T, Jung C et al (2017) The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS One 12:e0180181. https://doi.org/10.1371/journal.pone.0180181
Wu C, Dedhar S (2001) Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 155:505–510. https://doi.org/10.1083/jcb.200108077
Prasetyanti PR, Medema JP (2017) Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer 16(1):41. https://doi.org/10.1186/s12943-017-0600-4
Parton RG, Richards AA (2003) Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4:724–738
Wang Z, Wang N, Li W et al (2014) Caveolin-1 mediates chemoresistance in breast cancer stem cells via beta-catenin/ABCG2 signaling pathway. Carcinogenesis 35:2346–2356. https://doi.org/10.1093/carcin/bgu155
Moreno-Vicente R, Pavón DM, Martín-Padura I et al (2018) Caveolin-1 modulates mechanotransduction responses to substrate stiffness through actin-dependent control of YAP. Cell Rep 25(6):1622–1635.e6. https://doi.org/10.1016/j.celrep.2018.10.024
Acerbi I, Cassereau L, Dean I et al (2015) Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 7:1120–1134. https://doi.org/10.1039/c5ib00040h
Cordenonsi M, Zanconato F, Azzolin L et al (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147:759–772. https://doi.org/10.1016/j.cell.2011.09.048
Basu-Roy U, Bayin NS, Rattanakorn K et al (2015) Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun 6:6411. https://doi.org/10.1038/ncomms7411
Park JH, Shin JE, Park HW (2018) The role of Hippo pathway in cancer stem cell biology. Mol Cell 41:83–92. https://doi.org/10.14348/molcells.2018.2242
Triantafillu U et al (2017) Fluid shear stress induces cancer stem cell-like phenotype in MCF7 breast cancer cell line without inducing epithelial to mesenchymal transition. Int J Oncol 50:993–1001. https://doi.org/10.3892/ijo.2017.3865
Hieda M (2017) Implications for diverse functions of the LINC complexes based on the structure. Cells 6(1):3. https://doi.org/10.3390/cells6010003
Constantinescu D, Gray HL, Sammak PJ et al (2006) Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24:177–185. https://doi.org/10.1634/stemcells.2004-0159
Matsumoto A, Hieda M, Yokoyama Y et al (2015) Global loss of a nuclear lamina component, Lamin A/C, and LINC complex components SUN1, SUN2, and nesprin-2 in breast cancer. Cancer Med 4:1547–1557. https://doi.org/10.1002/cam4.495
Tan Y, Tajik A, Chen J et al (2014) Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun 5:717–728. https://doi.org/10.1038/ncomms5619
Chen J, Kumar S (2017) Biophysical regulation of cancer stem/initiating cells: implications for disease mechanisms and translation. Curr Opin Biomed Eng 1:87–95. https://doi.org/10.1016/j.cobme.2017.02.006
Xu X, Farach-Carson MC, Jia X (2014) Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol Adv 32(7):1256–1268. https://doi.org/10.1016/j.biotechadv.2014.07.009
Irimia D, Toner M (2009) Spontaneous migration of cancer cells under conditions of mechanical confinement. Integr Biol 1(8–9):506–512. https://doi.org/10.1039/b908595e
Gossett DR, Tse HTK, Lee SA et al (2012) Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A 109:7630–7635. https://doi.org/10.1073/pnas.1200107109
Darling EM, Di Carlo D (2015) High-throughput assessment of cellular mechanical properties. Annu Rev Biomed Eng 17:35–62. https://doi.org/10.1146/annurev-bioeng-071114-040545
Yongming Xu, Shujuan Xu, Zhi Gao et al. (2018) Degree of endplate chondrocyte degeneration in different tension regions during mechanical stimulation. Mol Med Rep 17(3):4415–4421. https://doi.org/10.3892/mmr.2018.8435
Ordikhani F, Kim Y, Zustiak SP (2015) The role of biomaterials on cancer stem cell enrichment and behavior. JOM 67(11):2543–2549. https://doi.org/10.1007/s11837-015-1626-y
Baker EL, Bonnecaze RT, Zaman MH (2009) Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J 97(4):1013–1021. https://doi.org/10.1016/j.bpj.2009.05.054
Serwane F, Mongera A, Rowghanian P et al (2009) In vivo quantification of spatially varying mechanical properties in developing tissues. Nat Methods 97(4):1013–1021. https://doi.org/10.1038/nmeth.4101
Barker HE, Bird D, Lang G, Erler JT (2013) Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res 11(11):1425–1436. https://doi.org/10.1158/1541-7786.MCR-13-0033-T
Venkatesh V, Nataraj R, Thangaraj GS et al (2018) Targeting notch signalling pathway of cancer stem cells. Stem Cell Investig 5:5. https://doi.org/10.21037/sci.2018.02.02
Lang T, Ding X, Kong L et al (2018) NFATC2 is a novel therapeutic target for colorectal cancer stem cells. Onco Targets Ther 11:6911–6924. https://doi.org/10.2147/OTT.S169129
Zhang S, Zhang H, Ghia EM et al (2019) Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci USA 116(4):1370–1377. https://doi.org/10.1073/pnas.1816262116
Cazet AS, Hui MN, Elsworth BL et al (2018) Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun 9:2897. https://doi.org/10.1038/s41467-018-05220-6
Acknowledgements
This study was supported by Intramural Research Grant (A-523) from AIIMS, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript does not contain clinical studies or patient data.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roy Choudhury, A., Gupta, S., Chaturvedi, P.K. et al. Mechanobiology of Cancer Stem Cells and Their Niche. Cancer Microenvironment 12, 17–27 (2019). https://doi.org/10.1007/s12307-019-00222-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-019-00222-4