Abstract
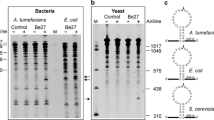
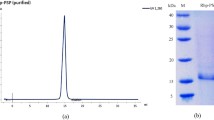
Considering Celosia plumosa as a potent antiviral plant, the attempt was made to determine, purify and characterize its proteinaceous antiviral elements against tobacco mosaic virus hypersensitive response on Nicotiana glutinosa. By using 60% ammonium sulphate-precipitation, FPLC-based anion and cation-exchange chromatography in 10 and 50 mM NaCl, size-exclusion chromatography in 50 mM NaCl and SDS–PAGE 10%, a 25 kD antiviral protein with ribosome-inactivating/28S rRNase ability was purified from the leaves of C. plumosa at vegetative growth stage. The purified protein showed FRAP-based antioxidant activity in vitro and caused 1.7-fold and 1.4-fold increases in the growth rate of root system upon carborundum-based application on the root growth medium of N. glutinosa. The present work reports an antiviral protein with ribosome-inactivating, antioxidation and root developer potencies in C. plumosa as an edible or ornamental plant that may be useful in health and agricultural biotechnology in the future.





Similar content being viewed by others
Abbreviations
- FRAP:
-
Ferric reducing antioxidant power
- AVP:
-
Antiviral proteins
- RIP:
-
Ribosome-inactivating proteins
- TMV:
-
Tobacco mosaic virus
- BME:
-
Beta mercaptoethanol
- PVP:
-
Polyvinylpyrrolidone
- FPLC:
-
Fast performance liquid chromatography
- SDS:
-
Sodium dodecyl sulphate
- EGTA:
-
Ethylene glycol tetraacetic acid
- DTT:
-
Dithiothreitol
- BSA:
-
Bovine serum albumin
References
Balasubrahmanyam A, Baranwal VK, Lodha ML, Varma A, Kapoor HC (2000) Purification and properties of growth stage dependent antiviral proteins from the leaves of Celosia cristata. Plant Sci 154:13–21
Barbieri L, Battelli MG, Stripe F (1993) Ribosome-inactivating proteins from plants. Biochem et Biophy Acta 1154:237–282
Battelli MG, Polito L, Bolognesi A, Lafleur L, Fradet Y, Stirpe F (1996) Toxicity of ribosome-inactivating proteins containing immunotoxins to a human bladder carcinoma cell line. Int J Cancer 65:485–490
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Gholizadeh A (2016) Potential involvement of maize ribosome-inactivating protein in root development. Bangladesh J Bot 45:1–8
Gholizadeh A, Kapoor HC (2004) Modifications in the purification protocol of Celosia cristata antiviral proteins leads to protein that can be N-terminally sequenced. Protein Pept Lett 11:555–561
Gholizadeh A, Pourrahim R (2017) Identification and differential biophysical characterization of antiviral potentials from different Celosia plants. Plant Cell Biotechnol Mol Biol 18:527–534
Gholizadeh A, Kumar M, Balasubramanyam A, Sharma S, Narwal ML, Kapoor HC (2004) Antioxidant activity of antiviral proteins from Celosia cristata L. Plant Biochem Biotechnol 13:13–18
Gholizadeh A, BaghbanKR B, Santha IM, Lodha ML, Kapoor HC (2005) Cloning and expression of a small cDNA fragment encoding a strong antiviral peptide from Celosia cristata in E. coli. Biochemistry 70:1005–1010
Girbes T, Ferreras JM, Arias FJ, Stripe F (2004) Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev Med Chem 4:461–476
Hartley MR, Lord JM (1993) Structure, function and applications of ricin and related cytotoxic proteins. In: Grierson D (ed) Biosynthesis and manipulation of plant products. Chapman and Hall, Glasgow, pp 210–239
He YW, Guo CX, Pan YF, Peng C, Weng ZH (2008) Inhibition of hepatitis B virus replication by pokeweed antiviral protein in vitro. World J Gastroenterol 14:1592–1597
Hong Y, Saunders K, Hartle MR, Stanley J (1996) Resistance to geminivirus infection by virus-induced expression of dianthin in transgenic plants. Virology 220:119–127
Iglesias R, Preze Y, de Torre C, Ferreras JM, Antolin P, Jimenez P, Rojo MA, Mendez E, Girbes T (2005) Molecular characterization and systemic induction of single-chain ribosome-inactivating proteins (RIPs) in sugar beet (Beta vulgaris) leaves. J Exp Bot 56:1675–1684
Irvin JD (1995) Antiviral protein from Phytolacca. In: Deborde D, Zipf A, Chessin M (eds) Antiviral proteins at higher plants. CRC Press, Boca Raton, pp 65–91
Kirtikar KR, Basu BD (1935) Indian medicinal plant, vol III, 2nd edn. Singh B, Mahender Pal Singh, Dehradun, pp 2053–2054
Knight B (1979) Ricin—a potent homicidal poison. Br Med J 1:350–351
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
National Research Council (2006) Lost crops of Africa, vegetables, vol II. The National Academies Home, Washington, pp 93–95
Pandit N, Singla RK, Shrivastava B (2013) Antiviral proteins from family Amaranthaceae: an overview. Indo Glob J Pharm Sci 3:192–197
Parikh BA, Tumer NE (2004) Antiviral activity of ribosome-inactivating proteins in medicine. Mini Rev Med Chem 4:523–543
Park SW, Christopher B, Lawrence Linden JC, Vivanco JM (2002) Isolation and characterization of a novel RIP from root cultures of pokeweed and its mechanism of secretion from roots. Plant Physiol 130:164–178
Park SW, Vepachedu R, Sharma N, Vivanco JM (2004) Ribosome-inactivating proteins in plant biology. Planta 219:1093–1096
Peumans WJ, Hao Q, Van Damme EJ (2001) Ribosome- inactivating proteins from plants: more than RNA N-glycosidases? FASEB J 15:1493–1506
Pizzo E, Antimo DM (2016) A new age for bomedical applications of ribosome inactivating proteins: from bioconjugate to nanoconstructs. J Biomed Sci 23:1–8
Poma A, Galeota K, Miranda M, Spano L (1997) A RIP principle from hairy roots and seeds of Luffa cylindrical (L) Roem and its cititoxicity on melanotic and amelanotic melanoma cell lines. Pharm Biol 35:212–214
Puri M, Kaur I, Kanwar RK, Gupta RC, Chauthan A, Kanwar JR (2009) Ribosome inactivating proteins (RIPs) from Momordica charantia for anti viral therapy. Curr Mol Med 9:1080–1094
Pyo YH, Yoon MY, Son JH, Cho TB (2008) The effect of Celosia cristata L. ethanol extract on anti-oxidant and anti-aging activity. Korean J Biotechnol Bioeng 23:431–438
Rubini D, Sudhahar D, Anandrajgopal K (2012) Phytochemical investigation and anthelmintic activity of Celosia cristata leaf extracts. Int Res J Pharm 3:335–337
Schrot J, Weng A, Melzig M (2015) Ribosome-inactivating and related proteins. Toxins 7:1556–1615
Shu SH, Xie GZ, Guo XL, Wang M (2009) Purification and characterization of a novel ribosome-inactivating protein from seeds of Trichosanthes kirilowii Maxim. Protein Expr Purif 67:120–125
Sobiya RO, Jannet VJ (2013) Progress in ribosome inactivating protein studies: recent review of potential applications. Int J Pharm Biol Sci 3:88–100
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68
Stripe F, Batteli MG (2006) Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci 63:1850–1866
Stripe F, Wiliams DG, Onyon LG, Legg RF (1981) Dianthus, ribosome inactivating proteins with antiviral properties from Dianthus caryophyllus. Biochem J 195:399–405
Surse SN, Shrivastava B, Sharma P, Gide PS, Sana A (2014) Celosia cristata: potent pharmacotherapeutic herb. Int J Pharm Phytopharm Res (eIJPPR) 3:1–10
Uckun FM, Rajamohan F, Pendergrass S (2003) Structure-based design and engineering of a nontoxic recombinant pokeweed antiviral protein with potent anti-human immunodeficiency virus activity. Antimicrob Agents Chemother 47:1052–1061
Veronese P, Ruiz MT, Coca MA (2003) In defense against pathogens. Both plant sentinels and foot soldiers need to know the enemy. Plant Physiol 131:1580–1590
Woo K, Ko J, Song S, Lee J, Kang J (2011) Antioxidant compounds and antioxidant activities of the methanolic extracts from Cockscome (Celosia cristata L.) flowers. Planta Med 77:77–78
Yeung HW, Li WW, Feng Z, Barbieri L, Stirpe F (1988) Trichosanthin, α-momorcharin and β-momorcharin: identity of abortifacient and ribosome-inactivating proteins. Int J Pept Protein Res 31:265–268
Acknowledgements
The author of this paper is thankful to Iran National Science Foundation (INSF), Tehran and Research Institute for Fundamental Sciences (RIFS), University of Tabriz, Iran for their funding and providing facilities for the present research work.
Funding
Iran National Science Foundation, Tehran, Iran (Grant No. 88001730).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gholizadeh, A. Purification of a ribosome-inactivating protein with antioxidation and root developer potencies from Celosia plumosa. Physiol Mol Biol Plants 25, 243–251 (2019). https://doi.org/10.1007/s12298-018-0577-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-018-0577-5