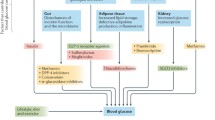
Abstract
Prevalence of diabetes mellitus, a chronic metabolic disease characterized by hyperglycemia, is growing worldwide. The majority of the cases belong to type 2 diabetes mellitus (T2DM). Globally, India ranks second in terms of diabetes prevalence among adults. Currently available classes of therapeutic agents are used alone or in combinations but seldom achieve treatment targets. Diverse pathophysiology and the need of therapeutic agents with more favourable pharmacokinetic-pharmacodynamics profile make newer drug discoveries in the field of T2DM essential. A large number of molecules, some with novel mechanisms, are in pipeline. The essence of this review is to track and discuss these potential agents, based on their developmental stages, especially those in phase 3 or phase 2. Unique molecules are being developed for existing drug classes like insulins, DPP-4 inhibitors, GLP-1 analogues; and under newer classes like dual/pan PPAR agonists, dual SGLT1/SGLT2 inhibitors, glimins, anti-inflammatory agents, glucokinase activators, G-protein coupled receptor agonists, hybrid peptide agonists, apical sodium-dependent bile acid transporter (ASBT) inhibitors, glucagon receptor antagonists etc. The heterogeneous clinical presentation and therapeutic outcomes in phenotypically similar patients is a clue to think beyond the standard treatment strategy.


Similar content being viewed by others
References
IDF diabetes atlas. International Diabetes Federation. 2015. http://www.diabetesatlas.org. Accessed 03 Aug 2016.
DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Res. 1997;5:177–269.
Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26(5):1408–12.
Jayant D, Lawrence B, Richard G. Factor influencing patient acceptability of diabetes treatment regimens. Clin Diabetes. 2000;18(2):61–7.
Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–41.
Currie CJ, Peyrot M, Morgan CL, Poole CD, Jenkins-Jones S, Rubin RR, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35:1279–84.
Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51.
Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–99.
Egan AG, Blind E, Dunder K, Graeff PA, Hummer BT, Bourcier T, et al. Pancreatic safety of incretin based drugs—FDA and EMA assessment. N Engl J Med. 2014;370:794–7.
Steiner S, Hompesch M, Pohl R, Simms P, Flacke F, Mohr T, et al. A novel insulin formulation with a more rapid onset of action. Diabetologia. 2008;51:1602–6.
Novo Nordisk files for regulatory approval of faster-acting insulin aspart in the US for the treatment of type 1 and 2 diabetes. Novo Nordisk. 2015. http://www.novonordisk.com/media/news-details.1972083.html. Accessed 04 Aug 2016.
Novo Nordisk files for regulatory approval of faster-acting insulin aspart in the EU for the treatment of type 1 and 2 diabetes. Novo Nordisk. 2015. http://www.novonordisk.com/media/news-details.1971141.html. Accessed 04 Aug 2016.
Mittermayer F, Caveney E, De Oliveira C, Gourgiotis L, Puri M, Tai L-J, et al. Addressing unmet medical needs in type 2 diabetes: a narrative review of drugs under development. Curr Diabetes Rev. 2015;11(1):17–31.
Bergenstal RM, Rosenstock J, Arakaki RF, Prince MJ, Qu Y, Sinha VP, et al. A randomized, controlled study of once-daily LY2605541, a novel long-acting basal insulin, versus insulin glargine in basal insulin-treated patients with type 2 diabetes. Diabetes Care. 2012;35:2140–7.
Rosenstock J, Bergenstal RM, Blevins TC, Morrow LA, Prince MJ, Qu Y, et al. Better glycemic control and weight loss with the novel long-acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care. 2013;36:522–8.
2015 Integrated report. Eli Lilly and company. 2015. https://www.lilly.com/_assets/pdf/integrated-report.pdf. Accessed 03 Aug 2016.
Taylor R, Magnusson I, Rothman DL, Cline GW, Caumo A, Cobelli C, et al. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J Clin Invest. 1996;97:126–32.
Singhal P, Caumo A, Carey PE, Cobelli C, Taylor R. Regulation of endogenous glucose production after a mixed meal in type 2 diabetes. Am J Physiol Endocrinol Metab. 2002;283:E275–83.
Zijlstra E, Heinemann L, Plum-Mörschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol. 2014;8(3):458–65.
Santos Cavaiola T, Edelman S. Inhaled insulin: a breath of fresh air? A review of inhaled insulin. Clin Ther. 2014;36:1275–89.
Zisser H, Jovanovic L, Markova K, Petrucci R, Boss A, Richardson P, et al. Technosphere insulin effectively controls postprandial glycemia in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2012;14:997–1001.
Ceglia L, Lau J, Pittas AG. Meta-analysis: efficacy and safety of inhaled insulin therapy in adults with diabetes mellitus. Ann Intern Med. 2006;145:665–75.
New drug application approval of zafatek® tablets for the treatment of type 2 diabetes in japan. Takeda Pharmaceutical Company Limited. 2015. https://www.takeda.com/news/2015/20150326_6953.html. Accessed 04 Aug 2016.
McKeage K. Trelagliptin: first global approval. Drugs. 2015;75:1161–4.
Inagaki N, Onouchi H, Sano H, Funao N, Kuroda S, Kaku K. SYR-472, a novel once weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:125–32.
Inagaki N, Onouchi H, Maezawa H, Kuroda S, Kaku K. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol. 2015;3:191–7.
Evans PM, Bain SC. Omarigliptin for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother. 2016;17(14):1947–52.
Merck provides update on filing plans for omarigliptin, an investigational DPP-4 inhibitor for type 2 diabetes. Merck & company. 2016. http://www.mercknewsroom.com/news/company-statements/merck-provides-update-filing-plans-omarigliptin-investigational-dpp-4-inhibi. Accessed 05 Aug 2016.
Evidence review of marizev, a pipeline DPP-4 treatment for type 2 diabetes. Advera health analytics. 2016. http://info.adverahealth.com/marizev-evidence-review. Accessed 06 Aug 2016.
Bunck MC, Cornér A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, et al. Effects of Exenatide on measures of β-cell function after 3 years in Metformin treated patients with type 2 diabetes. Diabetes Care. 2011;34:2041–7.
Drucker DJ. Deciphering metabolic messages from the gut drives therapeutic innovation: the 2014 Banting Lecture. Diabetes. 2015;64:317–26.
Novo Nordisk announces positive results for phase 2 trial with oral semaglutide in people with type 2diabetes. Novo Nordisk. 2015. http://www.novonordisk.com/media/news-details.1896081.html. Accessed 07 Aug 2016.
Novo Nordisk to initiate phase 3a development of oral semaglutide, a once-daily oral GLP-1 analogue. Novo Nordisk. 2015. http://www.novonordisk.com/media/news-details.1947638.html. Accessed 07 Aug 2016.
Novo Nordisk successfully completes fifth phase 3a trial with semaglutide in people with type 2 diabetes. Novo Nordisk. 2016. http://www.novonordisk.com/media/news-details.1988465.html. Accessed 11 Sept 2016.
Rohloff CM, Alessi TR, Yang B, Dahms J, Carr JP, Lautenbach SD. DUROS® technology delivers peptides and proteins at consistent rate continuously for 3 to 12 months. J Diabetes Sci Technol. 2008;2(3):461–7.
Henry RR, Rosenstock J, Logan D, Alessi T, Luskey K, Baron MA. Continuous subcutaneous delivery of exenatide via ITCA 650 leads to sustained glycemic control and weight loss for 48 weeks in metformin-treated subjects with type 2 diabetes. J Diabetes Complic. 2014;28(3):393–8.
Intarcia announces new top-line phase 3 results for investigational therapy ITCA 650 in type 2 diabetes: freedom-2 comparative trial demonstrates superior and sustained glucose control and weight reduction vs januvia over 52 weeks. Intarcia Therapeutics, Inc. 2016. http://www.intarcia.com/media/press-releases/2015-aug-18-freedom2.html. Accessed 15 Aug 2016.
ITCA 650. Intarcia Therapeutics, Inc. http://www.intarcia.com/pipeline-technology/itca-650.html. Accessed 07 Aug 2016.
Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35.
Henriksen K, Byrjalsen I, Qvist P, Beck-Nielsen H, Hansen G, Riis BJ, et al. Efficacy and safety of the PPARγ partial agonist balaglitazone compared with pioglitazone and placebo: a phase III, randomized, parallel-group study in patients with type 2 diabetes on stable insulin therapy. Diabetes Metab Res Rev. 2011;27(4):392–401.
Kim SG, Kim DM, Woo J-T, Jang HK, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS ONE. 2014;. doi:10.1371/journal.pone.00928439.
Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015;17(6):599–602.
Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294(20):2581–6.
AstraZeneca annual report 2006. AstraZeneca. 2007. https://www.astrazeneca.com/content/dam/az/PDF/2006_Annual_Report.pdf. Accessed 11 Aug 2016.
Roche halts investigation of aleglitazar following regular safety review of phase III trial. Roche. 2013. http://www.roche.com/media/store/releases/med-cor-2013-07-10.htm. Accessed 15 Aug 2016.
He BK, Ning ZQ, Li ZB, Shan S, Pan DS, Ko BCB, et al. In vitro and in vivo characterizations of chiglitazar, a newly identified ppar pan-agonist. PPAR Res. 2012;. doi:10.1155/2012/546548.
Li P-P, Shan S, Chen Y-T, Ning Z-Q, Sun S-J, Liu Q, et al. The PPAR α/γ dual agonist chiglitazar improves insulin resistance and dyslipidemia in MSG obese rats. Br J Pharmacol. 2006;148(5):610–8.
Phase IIa clinical trial of chiglitazar completed. Chipscreen biosciences. 2007. http://www.chipscreen.com/News/201309140810501322442224.html. Accessed 15 Aug 2016.
Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–42.
Hediger MA, Rhoads DB. Molecular physiology of sodium–glucose cotransporters. Physiol Rev. 1994;74:993–1026.
Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94.
Clinical trials: sotagliflozin (LX4211). Lexicon pharmaceuticals. 2016. http://www.lexpharma.com/pipeline/lx4211.html. Accessed 23 Aug 2016.
Vuylsteke V, Chastain LM, Maggu GA, Brown C. Imeglimin: a potential new multi-target drug for type 2 diabetes. Drugs R D. 2015;15(3):227–32.
Bays H, Mandarino L, DeFronzo RA. Role of the adipocytes, FFA, and ectopic fat in the pathogenesis of type 2 diabetes mellitus: PPAR agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–78.
Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, et al. Targeting inflammation using salsalate in type 2 diabetes study team: salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12.
Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Investig Drugs. 2015;24:283–307.
Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, Han Y, et al. The network of glucokinase- expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55:1–12.
Niswender KD, Shiota M, Postic C, Cherrington AD, Magnuson MA. Effects of increased glucokinase gene copy number on glucose homeostasis and hepatic glucose metabolism. J Biol Chem. 1997;272:22570–5.
Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–6.
Wilding JP, Leonsson-Zachrisson M, Wessman C, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 in patients with type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2013;15:750–9.
Tan T, Bloom S. Gut hormones as therapeutic agents in treatment of diabetes and obesity. Curr Opin Pharmacol. 2013;13:996–1001.
Mancini AD, Poitout V. GPR40 agonists for the treatment of type 2 diabetes: life after ‘TAKing’ a hit. Diabetes Obes Metab. 2015;17:622–9.
Bharate SB, Nemmani KV, Vishwakarma RA. Progress in the discovery and development of small-molecule modulators of G-protein-coupled receptor 40 (GPR40/FFA1/FFAR1): an emerging target for type 2 diabetes. Expert Opin Ther Pat. 2009;19:237–64.
Oh DY, Olefsky JM. G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat Rev Drug Discov. 2016;15:161–72.
Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Investig. 1967;46:1954–62.
Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8–12.
Pocai A. Unraveling oxyntomodulin, GLP1’s enigmatic brother. J Endocrinol. 2012;215:335–46.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5.
Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40.
Plaisancié P, Dumoulin V, Chayvialle JA, Cuber JC. Luminal glucagon-like peptide-1(7–36) amide-releasing factors in the isolated vascularly perfused rat colon. J Endocrinol. 1995;145:521–6.
Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780–6.
Adrian TE, Ballantyne GH, Longo WE, Bilchik AJ, Graham S, Basson MD, et al. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34(9):1219–24.
Nunez DJ, Yao X, Lin J, Walker A, Zuo P, Webster L, et al. Glucose and lipid effects of the ileal apical sodium-dependent bile acid transporter inhibitor GSK2330672: double-blind randomized trials with type 2 diabetes subjects taking metformin. Diabetes Obes Metab. 2016;18(7):654–62.
Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28(3):253–83.
Bagger JI, Knop FK, Holst JJ, Vilsboll T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab. 2011;13:965–71.
Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science. 1982;215(4536):1115–6.
Rohatgi N, Aly H, Marshall CA, McDonald WG, Kletzien RF, Colca JR, et al. Novel insulin sensitizer modulates nutrient sensing pathways and maintains β-cell phenotype in human islets. PLoS ONE. 2013. doi:10.1371/journal.pone.0062012.
Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100.
Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–6.
Van Poelje PD, Potter SC, Chandramouli VC, Landau BR, Dang Q, Erion MD. Inhibition of fructose 1, 6-biphosphatase reduces excessive endogenous glucose production and attenuates hyperglycemia in Zucker diabetic fatty rats. Diabetes. 2006;55:1747–54.
Denison H, Nilsson C, Kujacic M, Lofgren L, Karlsson C, Knutsson M, et al. Proof of mechanism for the DGAT1 inhibitor AZD7687: results from a first-time-in-human single-dose study. Diabetes Obes Metab. 2013;15:136–43.
Hollis G, Huber R. 11β-Hydroxysteroid dehydrogenase type 1 inhibition in type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13(1):1–6.
Anderson A, Walker BR. 11β-HSD1 inhibitors for the treatment of type 2 diabetes and cardiovascular disease. Drugs. 2013;73(13):1385–93.
Singh PK, Hota D, Dutta P, Sachdeva N, Chakrabarti A, Srinivasan A, et al. Pantoprazole improves glycemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2012;97(11):2105–8.
Vetere A, Choudhary A, Burns SM, Wagner BK. Targeting the pancreatic β-cell to treat diabetes. Nat Rev Drug Discov. 2014;13:278–89.
Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 2003;362:1275–81.
Zhou K, Donnelly L, Burch L, Tavendale R, Doney AS, Leese G, et al. Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther. 2010;87:52–6.
Ragia G, Petridis I, Tavridou A, Christakidis D, Manolopoulos VG. Presence of CYP2C9*3 allele increases risk for hypoglycemia in type 2 diabetic patients treated with sulfonylureas. Pharmacogenomics. 2009;10:1781–7.
Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CN, Pearson ER. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS study. Diabetes. 2015;64(5):1786–93.
Zhou K, Bellenguez C, Spencer CC, Bennett AJ, Coleman RL, Tavendale R, et al. Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet. 2011;43(2):117–20.
Acknowledgements
All authors have contributed significantly to a collection of information and designing and formatting of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chikara, G., Sharma, P.K., Dwivedi, P. et al. A Narrative Review of Potential Future Antidiabetic Drugs: Should We Expect More?. Ind J Clin Biochem 33, 121–131 (2018). https://doi.org/10.1007/s12291-017-0668-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-017-0668-z