Abstract
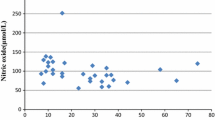
Oxidative stress contributes to the cascade, leading to dopamine cell degeneration in Parkinson’s disease. However, oxidative stress is intimately linked to other components of the degenerative process, such as mitochondrial dysfunction, excitotoxicity, nitric oxide toxicity and inflammation. It is therefore difficult to determine whether oxidative stress leads to or is a consequence of, these events. Oxidative stress was assessed by estimating lipid peroxidation product in the form of thiobarbituric acid reactive substances, nitric oxide in the form of nitrite & nitrate. Enzymatic antioxidants in the form of superoxide dismutase, glutathione peroxidase, catalase, ceruloplasmin and non enzymatic antioxidant vitamins e.g. vitamin E and C in either serum or plasma or erythrocyte in 40 patients of Parkinson’s disease in the age group 40–80 years. Trace elements e.g. copper, zinc and selenium were also estimated. Plasma thiobarbituric acid reactive substances and nitric oxide levels were Significantly high but superoxide dismutase, glutathione peroxidase, catalase, ceruloplasmin, vitamin-E, vitamin-C, copper, zinc and selenium levels were significantly low in Parkinson’s disease when compared with control subjects. Present study showed that elevated oxidative stress may be playing a role in dopaminergic neuronal loss in substentia nigra pars compacta and involved in pathogenesis of the Parkinson’s disease.
Similar content being viewed by others
References
Martin JB. Moleculor basis of the neurodegenerative disorder. New Eng J Med 1999; 340(25):1970–1980.
Pioro EP. Antioxidant therapy in ALS. Amyotrophic Lateral Scleorsis 2000; 1 (Supl): 5–15.
Kowall W, Ferante RJ, Martin JB. Patterns of cells in Huntington’s disease. Trends Neurosci 1987;10: 42–49.
Mmosley RL, Benner EJ, Irena. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neurochem Res 2006; 6(5):261–281.
Jellinger K. Pathology of Parkinson’s disease, changes other than the nigrostritalpathway. Ann Neural 1992; 14: 153–97.
Forne LS. Pathology of Parkinson’s disease and importance of substentia nigra and Lewy bodies. Aetiology of Parkinson’s disease. Ed. Sterm. G. Baltimore Johns Hopkins Press: 1990; p 185–238.
Kurtz JF. An overview of epidemiology of Parkinsonism. Progress inParkinson’s disease research. Eds. Hefti F. Weiner W.J. Mount Kisw Futura Publishing Company. 1992; p-119–p-169.
Munch G. Oxidative stress and advanced glycation end products. Alzheimer’s Disease Review 1996;1:71–74.
Benecke R, Strumper P, Weiss H. Electron transport complex CI & IV of platelets are abnormal in Parkinson’s disease. Brain 1993;116;1451–1463.
Buege JA, Aust AD. Microsomal lipid peroxidation.In methods Enzymeol Estbrook RW, Pullman ME. New York: Acad Press 1987;302–310
Cortas, Walkid. Determination of nitrate in serum and urine by kinetic cadmium reduction method. Clin Chem 1990;38(8pt.1); 1440–1443.
Randox Ltd. Determination of superoxide dismutase & Glutathione peroxide. Tech Bull on free radical 1994; 12–14.
Aebi H. Catalase. In methods, in enzymatic analysis (ed) Bergmeryer HU. Vol. 3. Acadamic press New York 1983; 276–286.
Karl H, Smith BSW, Wright H. Colorimetric method for serum Ceruloplasmin. Clin Chem 1974; 50: 359–366.
Baker Frank. Vitamin E. In clinical vitaminology methods & interpretation, Interscience publishers, New York 1968; 169–176.
Natelson S. Ascorbic acid. In techniques of clinical chemistry, 3rd Edition, Charles C Thomas, Illinios USA 1971;162–165.
Fahn S. Parkinson’s disease and other basal ganglion disorders. IN: Disease of nervous system. Clin Neurol. Eds.: Asbury AK et. al. W.B. Saunders company. 1992; 1144–1158.
Ratan RR, Baraban JM. Apoptotic death in an invitro model of neuronal oxidative stress. Clin Ext Pharmacol Physical 1995; 309–310.
Mecocci. The oxidative damage mitochondrial DNA shows age dependent increase in human brain. Ann Neural 1993:34; 159–163.
Floryed RA Antioxidants, oxidative stress and degenerative neurological disorders. Proc Soc Exper Biol Med 1999; 222(3): 236–245
Behl C, Davis J, Cole GM. Vitamin E protects nerves cells from amyloid beta protein toxicity. Biochem Biophy Res Commun 1992; 186: 944–950.
Brown R. ALS and the inherited motor neurons disease. Mol Neurol. New York Scientific Americam 1998; 223:38.
Burke RE. Apoptosis in degenerative disease of the basal ganglia. Neurosciertist 1998; 4:301–311.
Bhumik D, Medin J, Colmenms G. Mutational analysis of active site residue of human adenosine deaminase. J Biol Chem 1993; 8268: 5461–5470.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikam, S., Nikam, P., Ahaley, S.K. et al. Oxidative stress in Parkinson’s disease. Indian J Clin Biochem 24, 98–101 (2009). https://doi.org/10.1007/s12291-009-0017-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-009-0017-y