Abstract
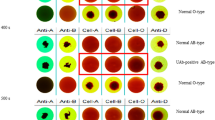
The automated immunohematology analyzer IH-500 (Bio-rad, Cressier FR, Switzerland) was developed recently for blood bank tests and this study evaluated performance of IH-500. 200 blood samples for ABO/Rh typing were collected. ABO/Rh typing results measured by IH-500 was compared with conventional manual methods. Antibody screening tests were performed with 100 samples using both IH-500 and the Ortho BioVue System, and results were compared. Antibody identification tests were conducted on 5 samples using both IH-500 and the Ortho BioVue System and results were compared. Crossmatching was performed with both IH-500 and conventional manual tube method using 4 patient serum samples and 10 blood cell donors, and 40 results were compared. Isoagglutinin titer of anti-A and anti-B was determined in 10 samples using both IH-500 and the automated analyzer Ortho AutoVue Innova and concordance rates were obtained. The concordance rates of ABO/Rh typing, antibody screening test, antibody identification test, and crossmatching between comparative manual methods and the IH-500 were all 100%. In the evaluation of isoagglutinin titer, 8 (80.0%) results out of 10 samples (80%) showed results within ± 1 titer between the IH-500 and the AutoVue Innova, which indicates the concordance rates of 80.0%. IH-500 reported results with two titers lower than Ortho AutoVue Innova in two samples. The IH-500 demonstrated good concordance rates and provided reliable results compared to comparative manual methods in the blood bank testing. IH-500 would be useful as a possible replacement for conventionally performed manual methods in blood bank testing.
Similar content being viewed by others
References
Brown MR, Fritsma MG, Marques MB (2005) Transfusion safety: what has been done; what is still needed? MLO Med Lab Obs 37(11):20–24
Davies A, Staves J, Kay J, Casbard A, Murphy MF (2006) End-to-end electronic control of the hospital transfusion process to increase the safety of blood transfusion: strengths and weaknesses. Transfusion 46(3):352–364
Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brand L et al (2006) Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev 20(4):273–282
Klouche M (2008) Editorial: impact of automation for testing in transfusion medicine and blood banking. Laboratoriumsmedizin 32(2):57–58
Dada A, Beck D, Schmitz G (2007) Automation and data processing in blood banking using the ortho AutoVue® Innova System. Transfus Med Hemother 34:341–346
Barkham TM (2004) Laboratory safety aspects of SARS at biosafety level 2. Ann Acad Med Singapore 33(2):252–256
Butch SH (2008) Automation in the transfusion service. Immunohematology 24(3):86–92
Sandler SG, Langeberg A, Avery N, Mintz PD (2000) A fully automated blood typing system for hospital transfusion services. ABS2000 study group. Transfusion 40(2):201–207
Shin SY, Kwon KC, Koo SH, Park JW, Ko CS, Song JH et al (2008) Evaluation of two automated instruments for pre-transfusion testing: AutoVue Innova and Techno TwinStation. Korean J Lab Med 28(3):214–220
Schoenfeld H, Pretzel KJ, von Heymann C, Neuner B, Kalus U, Kiesewetter H et al (2010) Validation of a hospital-laboratory workstation for immunohematologic methods. Transfusion 50(1):26–31
Taylor J, Hyare J, Stelfox P, Williams M, Lees R, Maley M (2011) Multi-centre evaluation of pre-transfusional routine tests using 8-column format gel cards (DG Gel®). Transfus Med 21(2):90–98
Shin KH, Kim HH, Chang CL, Lee EY (2013) Economic and workflow analysis of a blood bank automated system. Ann Lab Med 33(4):268–273
Chang C, Brown M, Davies L, Pointon L, Brown R, Barker D (2014) Evaluation of Erytra® fully automated analyser for routine use in transfusion laboratory. Transfus Med 24(1):33–38
Cheng YW, Wilkinson JM (2015) An experience of the introduction of a blood bank automation system (Ortho AutoVue Innova) in a regional acute hospital. Transfus Apher Sci 53(1):58–63
Roback JD, Barclay S, Moulds JM, Denomme GA (2015) A multicenter study on the performance of a fully automated, walk-away high-throughput analyzer for pretransfusion testing in the US population. Transfusion 55:1522–1528
Shin JW, Shin WY, Lee DL (2017) Comparison of ABO blood group typing between automated blood bank analyzer IH-500 and manual method. Korean J Blood Transfus 28(2):126–133
Park YM, Kim SY, Koo SH, Lim J, Kim JM, Lim YA (2017) Evaluation of the automated blood bank systems IH-500 and VISION max for ABO-RhD blood typing and unexpected antibody screening. Lab Med Online 7(4):170–175
Morelati F, Revelli N, Maffei LM, Poretti M, Santoro C, Parravicini A (1998) Evaluation of a new automated instrument for pretransfusion testing. Transfusion 38(10):959–965
Funding
All authors declare that there is no funding regarding to this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, S.H., Kim, J., Lim, JH. et al. Performance Evaluation of Automated Immunohematology Analyzer IH-500 for Blood Bank Testing. Indian J Hematol Blood Transfus 35, 731–735 (2019). https://doi.org/10.1007/s12288-019-01127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01127-4