Abstract
Background
The primary objective of this multicenter, open-label, randomized, parallel, phase II selection trial was to compare the objective tumor response to biweekly (every 2 weeks) gemcitabine/paclitaxel, gemcitabine/carboplatin, and gemcitabine/cisplatin as first-line treatment for metastatic breast cancer.
Patients and methods
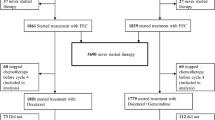
Eligible patients with stage IV disease who relapsed after anthracycline failure were randomly assigned in a 1:1:1 ratio to gemcitabine (2,500 mg/m2) plus paclitaxel 150 mg/m2 (n = 49); plus carboplatin, area under the curve = 2.5 mg/mL × min (n = 47); or plus cisplatin 50 mg/m2 (n = 51). Study therapy continued up until a maximum of 8 cycles and follow-up continued for 24 months.
Results
All patients were analyzed for efficacy and one patient was excluded from the safety analyses. The objective response was 26.5% [95% confidence interval (CI) 14.9–41.1] for gemcitabine/paclitaxel, 17.0% (95% CI 7.6–30.8) for gemcitabine/carboplatin, and 15.7% (95% CI 7.0–28.6) for gemcitabine/cisplatin. The adjusted odds ratio for tumor response was 0.33 (95% CI 0.10–1.06), P = 0.063 for gemcitabine/carboplatin versus gemcitabine/paclitaxel; 0.26 (95% CI 0.08–0.86), P = 0.027 for gemcitabine/cisplatin versus gemcitabine/paclitaxel; and 0.77 (95% CI 0.24–2.52), P = 0.671 for gemcitabine/cisplatin versus gemcitabine/carboplatin. There were no significant differences in overall survival or progression-free survival (P > 0.05). Grade 3 or 4 drug-related adverse events varied between groups and the majority of deaths (94.9%; 74/78) were related to disease progression.
Conclusions
The gemcitabine-based treatments had comparable activity and tolerability. Similar survival characteristics and different toxicity profiles suggested that gemcitabine–platinum may be evaluated further in patients after anthracycline failure.


Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Colozza M, de Azambuja E, Personeni N, Lebrun F, Picart M, Cardoso F. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007;12:253–70.
O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–9.
Cardoso F, Bedard P, Winer E, Paganini O, Senkus-Konefka E, Fallowfield L, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174–81.
Jassem J, Carroll C, Ward S, Simpson E, Hind D. The clinical efficacy of cytotoxic agents in locally advanced or metastatic breast cancer patients pretreated with an anthracycline and a taxane: a systematic review. Eur J Cancer. 2009;45:2749–58.
Dent S, Messersmith H, Trudeau M. Gemcitabine in the management of metastatic breast cancer: a systematic review. Breast Cancer Res Treat. 2008;108:319–31.
Perez EA. Gemcitabine and platinum combinations in patients with breast cancer previously treated with anthracyclines and/or taxanes. Clin Breast Cancer. 2004;4(Suppl 3):S113–6.
Yardley DA. Gemcitabine plus paclitaxel in breast cancer. Semin Oncol. 2005;32:S14–21.
Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan J, Llombart A, Pluzanska A, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26:3950–7.
Khoo K, Manzoor Zaidi S, Srimuninnimit V, Song S, Nair R, Ngelangel C, et al. Gemcitabine and split-dose paclitaxel or docetaxel in metastatic breast cancer: a randomised phase II study. Eur J Cancer. 2006;42:1797–806.
Allouache D, Gawande S, Tubiana-Hulin M, Tubiana-Mathieu N, Piperno-Neumann S, Mefti F, et al. First-line therapy with gemcitabine and paclitaxel in locally, recurrent or metastatic breast cancer: a phase II study. BMC Cancer. 2005;5:151.
Burch P, Mailliard J, Hillman D, Perez E, Krook J, Rowland K, et al. Phase II study of gemcitabine plus cisplatin in patients with metastatic breast cancer: a North Central Cancer Treatment Group Trial. Am J Clin Oncol. 2005;28:195–200.
Chan D, Yeo WL, Tiemsim Cordero M, Wong C, Chuah B, Soo R, et al. Phase II study of gemcitabine and carboplatin in metastatic breast cancers with prior exposure to anthracyclines and taxanes. Invest New Drugs. 2010;28:859–65.
Chew HK, Doroshow JH, Frankel P, Margolin K, Somlo G, Lenz H, et al. Phase II studies of gemcitabine and cisplatin in heavily and minimally pretreated metastatic breast cancer. J Clin Oncol. 2009;27:2163–9.
Colomer R, Llombart-Cussac A, Lluch A, Barnadas A, Ojeda B, Carañana V, et al. Biweekly paclitaxel plus gemcitabine in advanced breast cancer: phase II trial and predictive value of HER2 extracellular domain. Ann Oncol. 2004;15:201–6.
Delfino C, Caccia G, Riva Gonzáles L, Mickiewicz E, Rodger J, Balbiani L, et al. Gemcitabine/paclitaxel as first-line treatment of advanced breast cancer. Oncology (Williston Park). 2003;17:22–5.
Demiray M, Kurt E, Evrensel T, Kanat O, Arslan M, Saraydaroglu O, et al. Phase II study of gemcitabine plus paclitaxel in metastatic breast cancer patients with prior anthracycline exposure. Cancer Invest. 2005;23:386–91.
Fuentes H, Calderillo G, Alexander F, Ramirez M, Avila E, Perez L, et al. Phase II study of gemcitabine plus cisplatin in metastatic breast cancer. Anticancer Drugs. 2006;17:565–70.
Heinemann V, Stemmler HJ, Wohlrab A, Bosse D, Losem C, Kahlert S, et al. High efficacy of gemcitabine and cisplatin in patients with predominantly anthracycline- and taxane-pretreated metastatic breast cancer. Cancer Chemother Pharmacol. 2006;57:640–6.
Kim JH, Oh SY, Kwon HC, Lee S, Kim S, Kim D, et al. Phase II study of gemcitabine plus cisplatin in patients with anthracycline- and taxane- pretreated metastatic breast cancer. Cancer Res Treat. 2008;40:101–5.
Laessig D, Stemmler HJ, Vehling-Kaiser U, Fasching P, Melchert F, Kölbl H, et al. Gemcitabine and carboplatin in intensively pretreated patients with metastatic breast cancer. Oncology. 2007;73:407–14.
Mohran TZ. Gemcitabine and cisplatin combination chemotherapy as a first-line treatment in patients with metastatic breast cancer. J Egypt Natl Cancer Inst. 2004;16:8–14.
Nagourney RA, Flam M, Link J, Hager S, Blitzer J, Lyons W, et al. Carboplatin plus gemcitabine repeating doublet therapy in recurrent breast cancer. Clin Breast Cancer. 2008;8:432–5.
Nagourney RA, Link JS, Blitzer JB, Forsthoff C, Evans S. Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients. J Clin Oncol. 2000;18:2245–9.
Nasr FL, Chahine GY, Kattan JG, Farhat F, Mokaddem W, Tueni E, et al. Gemcitabine plus carboplatin combination therapy as second-line treatment in patients with relapsed breast cancer. Clin Breast Cancer. 2004;5:117–22.
Seo J, Oh S, Choi C, Kim B, Shin S, Kim Y, et al. Phase II study of a gemcitabine and cisplatin combination regimen in taxane resistant metastatic breast cancer. Cancer Chemother Pharmacol. 2007;59:269–74.
Somali I, Alacacioglu A, Tarhan MO, Meydan N, Erten C, Usalp S, et al. Cisplatin plus gemcitabine chemotherapy in taxane/anthracycline-resistant metastatic breast cancer. Chemotherapy. 2009;55:155–60.
Tas F, Guney N, Derin D, Camlica H, Aydiner A, Topuz E. Biweekly administration of gemcitabine and cisplatin chemotherapy in patients with anthracycline and taxane-pretreated metastatic breast cancer. Invest New Drugs. 2008;26:363–8.
Tomao S, Romiti A, Tomao F, Di Seri M, Caprio G, Spinelli G, et al. A phase II trial of a biweekly combination of paclitaxel and gemcitabine in metastatic breast cancer. BMC Cancer. 2006;6:137.
Yardley DA, Burris HA 3rd, Simons L, Spigel D, Greco F, Barton J, et al. A phase II trial of gemcitabine/carboplatin with or without trastuzumab in the first-line treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2008;8:425–31.
Fleming ID, Cooper JS, Henson DE, et al., eds. AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven; 1997.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
National Cancer Institute Cancer Therapy Evaluation Program. US National Institutes of Health. Common Toxicity Criteria (CTC), version 2.0. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_20. Accessed 5 March 2010.
Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez E, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76.
Oakman C, Viale G, Di Leo A. Management of triple negative breast cancer. Breast. 2010;19(5):312–21.
Acknowledgments
This study was sponsored by Eli Lilly and Company. The sponsor was involved in the design of the study, data collection and analysis, and the preparation of the manuscript, and did not impose any impediment, directly or indirectly, on the publication of the study’s results. The authors acknowledge the independent medical writing assistance provided by Serina Stretton (PhD) and Mark Woolley (PhD) of ProScribe Medical Communications, which was funded from an unrestricted financial grant from Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2). The authors would also like to thank all of the investigative site staff and patients who participated in the study, and Susanna Holt (Eli Lilly) for her assistance with the statistical analyses and editorial support. Part of this work was previously presented in abstract form at the 43rd Annual Meeting of the American Society of Clinical Oncology; 1–5 June 2007 (abstract #1099).
Conflict of interest
Daniel E. Lee Kay Pen is a full-time employee of Eli Lilly, the manufacturer of gemcitabine. Li Jun Shen was an employee of Eli Lilly while the study was being conducted and when the study data was analyzed. All of the other authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, B., Jiang, Z., Kim, SB. et al. Biweekly gemcitabine–paclitaxel, gemcitabine–carboplatin, or gemcitabine–cisplatin as first-line treatment in metastatic breast cancer after anthracycline failure: a phase II randomized selection trial. Breast Cancer 18, 203–212 (2011). https://doi.org/10.1007/s12282-011-0260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-011-0260-y