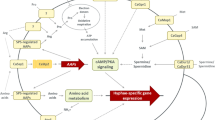
Abstract
Understanding which fungal factors allow colonization and infection of a human host is critical to lowering the incidence of human mycoses and related mortalities. In the pathogen Aspergillus fumigatus, secondary metabolites, small bioactive molecules produced by many opportunistic fungal pathogens, have important roles in suppressing and providing protection from host defenses. Deletion of LaeA, a global regulator of secondary metabolism in fungi, significantly decreases A. fumigatus virulence, in part owing to loss of gliotoxin and hydrophobin production. In addition to gliotoxin, dihydroxynaphthalene (DHN) melanin and siderophores are other A. fumigatus virulence factors; all three metabolites are derived from hallmark secondary metabolite gene clusters. Many of the gene clusters producing toxin metabolites have yet to be deciphered, and the study of secondary metabolites and their role in the virulence of human pathogens is a nascent field.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Davies J: Recombinant DNA and the Production of Small Molecules. Washington, DC: ASM Press; 1985.
Rohlfs M, Albert M, Keller NP, Kempken F: Secondary chemicals protect mould from fungivory. Biol Lett 2007, 3:523–525.
Proctor RH, Hohn TM, McCormick SP: Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact 1995, 8:593–601.
Paterson RR, Lima N: Toxicology of mycotoxins. EXS 2010, 100:31–63.
Richard JL: Some major mycotoxins and their mycotoxicoses—an overview. Int J Food Microbiol 2007, 119:3–10.
• Cichewicz RH: Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat Prod Rep 2009, 27:11–22. This article reviews the manipulation of chromatin-modifying enzymes to upregulate cryptic gene clusters and identify the resultant secondary metabolites.
Brase S, Encinas A, Keck J, Nising CF: Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 2009, 109:3903–3990.
Hoffmeister D, Keller NP: Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep 2007, 24, 393–416.
Nierman WC, Pain A, Anderson MJ, et al.: Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 2005, 438:1151–1156.
Maicas S, Moreno I, Nieto A, et al.: In silico analysis for transcription factors with Zn(II)2C6 binuclear cluster DNA-binding domains in Candida albicans. Comp Funct Genomics 2005, 6:345–356.
Bok JW, Chung D, Balajee SA, et al.: GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun 2006, 74:6761–6768.
Woloshuk CP, Foutz KR, Brewer JF, et al.: Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl Environ Microbiol 1994, 60:2408–2414.
Proctor RH, Hohn TM, McCormick SP, Desjardins AE: Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl Environ Microbiol 1995, 61:1923–1930.
Yu JH, Keller N: Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol 2005, 43:437–458.
Bok JW, Keller NP: LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell 2004, 3:527–535.
Bok JW, Balajee SA, Marr KA, et al.: LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell 2005, 4:1574–1582.
Kale SP, Milde L, Trapp MK, et al.: Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet Biol 2008, 45:1422–1429.
Fedorova ND, Khaldi N, Joardar VS, et al.: Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet 2008, 4:e1000046.
Kosalkova K, Garcia-Estrada C, Ullan RV, et al.: The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie 2009, 91:214–225.
Bayram O, Krappmann S, Ni M, et al.: VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320:1504–1506.
Amaike S, Keller NP: Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot Cell 2009, 8:1051–1060.
Perrin RM, Fedorova ND, Bok JW, et al.: Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog 2007, 3:e50.
Stinnett SM, Espeso EA, Cobeno L, et al.: Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol Microbiol 2007, 63:242–255.
Bok JW, Noordermeer D, Kale SP, Keller NP: Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol Microbiol 2006, 61:1636–1645.
Reyes-Dominguez Y, Bok JW, Berger H, et al.: Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol Microbiol 2010, 76:1376–1386.
Shwab EK, Bok JW, Tribus M, et al.: Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot Cell 2007, 6:1656–1664.
Dagenais TR, Giles SS, Aimanianda V, et al.: Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer. Infect Immun 2010, 78:823–829.
Sugui JA, Pardo J, Chang YC, et al.: Role of laeA in the Regulation of alb1, gliP, conidial morphology, and virulence in Aspergillus fumigatus. Eukaryot Cell 2007, 6:1552–1561.
Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP: Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009, 114:5393–5399.
• Aimanianda V, Bayry J, Bozza S, et al.: Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460:1117–1121. This paper demonstrates that the RodA protein coating the conidia surface renders the spores immunologically inert.
McDonagh A, Fedorova ND, Crabtree J, et al.: Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog 2008, 4:e1000154.
Menzel AEO, Wintersteiner O, Hoogerheide JC: The isolation of gliotoxin and fumigacin from culture filtrates of Aspergillus fumigatus. J Biol Chem 1944, 152:419–429.
Gardiner DM, Howlett BJ: Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol Lett 2005, 248:241–248.
Balibar CJ, Walsh CT: GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochemistry 2006, 45:15029–15038.
Cramer RA Jr, Gamcsik MP, Brooking RM, et al.: Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell 2006, 5:972–980.
Golden MC, Hahm SJ, Elessar RE, et al.: DNA damage by gliotoxin from Aspergillus fumigatus. An occupational and environmental propagule: adduct detection as measured by 32P DNA radiolabelling and two-dimensional thin-layer chromatography. Mycoses 1998, 41:97–104.
Kupfahl C, Geginat G, Hof H: Gliotoxin-mediated suppression of innate and adaptive immune functions directed against Listeria monocytogenes. Med Mycol 2006, 44(7):591–599.
Spikes S, Xu R, Nguyen CK, et al.: Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis 2008, 197:479–486.
Kupfahl C, Heinekamp T, Geginat G, et al.: Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol 2006, 62:292–302.
Sugui JA, Pardo J, Chang YC, et al.: Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 2007, 6:1562–1569.
• Kwon-Chung KJ, Sugui JA: What do we know about the role of gliotoxin in the pathobiology of Aspergillus fumigatus? Med Mycol 2009, 47(Suppl 1):S97–S103. This article reviews the equivocal results of five studies concerning gliotoxin as a virulence factor in mouse models and highlights that the immune suppression treatment used was the determining factor in all studies.
• Liu GY, Nizet V: Color me bad: microbial pigments as virulence factors. Trends Microbiol 2009, 17:406–413. This paper reviews a broad range of pigments and their roles in the virulence of bacteria, fungi, and protozoa pathogens.
• Karkowska-Kuleta J, Rapala-Kozik M, Kozik A: Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim Pol 2009, 56:211–224. This article reviews molecular mechanisms of virulence in addition to secondary metabolism for three important fungal pathogens.
Gomez BL, Nosanchuk JD: Melanin and fungi. Curr Opin Infect Dis 2003, 16:91–96.
Keller NP, Hohn TM: Metabolic pathway gene clusters in filamentous fungi. Fungal Genet Biol 1997, 21:17–29.
Fujii I, Mori Y, Watanabe A, et al.: Heterologous expression and product identification of Colletotrichum lagenarium polyketide synthase encoded by the PKS1 gene involved in melanin biosynthesis. Biosci Biotechnol Biochem 1999, 63:1445–1452.
Tsai HF, Chang YC, Washburn RG, et al: The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol 1998, 180:3031–3038.
Sugareva V, Hartl A, Brock M, et al.: Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch Microbiol 2006, 186:345–355.
• Chai LY, Netea MG, Sugui J, et al.: Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 2009 Nov 23 (Epub ahead of print). This paper advances our understanding of how melanins protect conidia from host defenses.
Tsai HF, Wheeler MH, Chang YC, et al.: A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 1999, 181:6469–6477.
•• Haas H, Eisendle M, Turgeon BG: Siderophores in fungal physiology and virulence. Annu Rev Phytopathol 2008, 46:149–187. This is a thorough review of iron acquisition mechanisms, siderophore biosynthesis, iron storage, iron metabolism regulation, and virulence in both plants and animals.
Schrettl M, Bignell E, Kragl C, et al.: Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog 2007, 3:1195–1207.
Wallner A, Blatzer M, Schrettl M, et al.: Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigatus. Appl Environ Microbiol 2009, 75:4194–4196.
Maiya S, Grundmann A, Li X, et al.: Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus. Chembiochem 2007, 8:1736–1743.
Maiya S, Grundmann A, Li SM, et al.: The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem 2006, 7:1062–1069.
Unsold IA, Li SM: Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1. Chembiochem 2006, 7:158–164.
Mitsuguchi H, Seshime Y, Fujii I, et al.: Biosynthesis of steroidal antibiotic fusidanes: functional analysis of oxidosqualene cyclase and subsequent tailoring enzymes from Aspergillus fumigatus. J Am Chem Soc 2009, 131:6402–6411.
Frisvad JC, Rank C, Nielsen KF, Larsen TO: Metabolomics of Aspergillus fumigatus. Med Mycol 2009, 47(Suppl 1):S53–S71.
Reeves EP, Reiber K, Neville C, et al.: A nonribosomal peptide synthetase (Pes1) confers protection against oxidative stress in Aspergillus fumigatus. FEBS J 2006, 273:3038–3053.
Kremer A, Westrich L, Li SM: A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 2007, 153:3409–3416.
Lodeiro S, Xiong Q, Wilson WK, et al.: Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus. Org Lett 2009, 11:1241–1244.
Ames BD, Walsh CT: Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry 2010, 49:3351–3365.
Pihet M, Vandeputte P, Tronchin G, et al.: Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol 2009, 9:177.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garvey, G.S., Keller, N.P. Fungal Secondary Metabolites and Their Fundamental Roles in Human Mycoses. Curr Fungal Infect Rep 4, 256–265 (2010). https://doi.org/10.1007/s12281-010-0032-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-010-0032-8