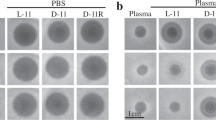
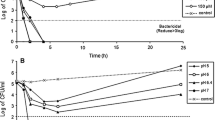
Abstract
Gram-negative bacteria secrete outer membrane vesicles (OMVs) that play critical roles in intraspecies, interspecies, and bacteria-environment interactions. Some OMVs, such as those produced by Pseudomonas aeruginosa, have previously been shown to possess antimicrobial activity against competitor species. In the current study, we demonstrate that OMVs from Burkholderia thailandensis inhibit the growth of drug-sensitive and drug-resistant bacteria and fungi. We show that a number of antimicrobial compounds, including peptidoglycan hydrolases, 4-hydroxy-3-methyl-2-(2-non-enyl)-quinoline (HMNQ) and long-chain rhamnolipid are present in or tightly associate with B. thailandensis OMVs. Furthermore, we demonstrate that HMNQ and rhamnolipid possess antimicrobial and antibiofilm properties against methicillin-resistant Staphylococcus aureus (MRSA). These findings indicate that B. thailandensis secretes antimicrobial OMVs that may impart a survival advantage by eliminating competition. In addition, bacterial OMVs may represent an untapped resource of novel therapeutics effective against bio-film-forming and multidrug-resistant organisms.
Similar content being viewed by others
References
Alves, N.J., Turner, K.B., Medintz, I.L., Walper, S.A. 2016. Protecting enzymatic function through directed packaging into bacterial outer membrane vesicles. Sci. Rep.6, 24866.
Beveridge, T.J. 1999. Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol.181, 4725–4733.
Beveridge, T.J., Makin, S.A., Kadurugamuwa, J.L., and Li, Z. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev.20, 291–303.
Biggins, J.B., Gleber, C.D., Brady, S.F. 2011. Acyldepsipeptide HDAC inhibitor production induced in Burkholderia thailandensis. Org. Lett.13, 1536–1539.
Biggins, J.B., Ternei, M.A., Brady, S.F. 2012. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J. Am. Chem. Soc.134, 13192–13195.
Bonnington, K.E. Kuehn, M.J. 2014. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta1843, 1612–1619.
Brett, P.J., DeShazer, D., Woods, D.E. 1998. Burkholderia thai-landensis sp. nov., a Burkholderia pseudomallei-Wke species. Int. ]. Syst. Bacteriol.48, 317–320.
Calfee, M.W., Shelton, J.G., McCubrey, J.A., Pesci, E.C. 2005. Solubility and bioactivity of the Pseudomonas quinolone signal are increased by a Pseudomonas aeruginosa-produced surfactant. Infect. Immun.73, 878–882.
Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centres for Disease Control and Prevention, Atlanta, Georgia, USA.
Costa, S.G.V.A.O., Deziel, E., and Lepine, F. 2011. Characterization of rhamnolipid production by Burkholderia glumae. Lett. Appl. Microbiol.53, 620–627.
Davies, D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. DrugDiscov.2, 114–122.
De Rienzo, M.A. Martin, P.J. 2016. Effect of mono and dirhamnolipids on biofilms pre-formed by Bacillus subtilis BBK006. Curr. Microbiol.73, 183–189.
Deatherage, B.L. Cookson, B.T. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun.80, 1948–1957.
DeFrancesco, A.S., Masloboeva, N., Syed, A.K., DeLoughery, A., Bradshaw, N., Li, G.W., Gilmore, M.S., Walker, S., and Losick, R. 2017. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA114, E5969–E5978.
Deziel, E., Lepine, F., Milot, S., He, J., Mindrinos, M.N., Tompkins, R.G., and Rahme, L.G. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA101, 1339–1344.
Dorward, D.W. Garon, C.F. 1990. DNA is packaged within membrane-derived vesicles of Gram-negative but not Gram-positive bacteria. Appl. Environ. Microbiol.56, 1960–1962.
Dubeau, D., Deziel, E., Woods, D.E., and Lepine, F. 2009. Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC Microbiol.9, 263.
Elshikh, M., Funston, S., Chebbi, A., Ahmed, S., Marchant, R., Banat, I.M. 2017. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. N. Biotechnol.36, 26–36.
Evans, A.G.L., Davey, H.M., Cookson, A., Currinn, H., Cooke-Fox, G., Stanczyk, P.J., Whitworth, D.E. 2012. Predatory activity of Myxococcus xanthus outer-membrane vesicles and properties of their hydrolase cargo. Microbiology158, 2742–2752.
Fukushima, T. and Sekiguchi, J. 2016. Zymographic techniques for the analysis of bacterial cell wall in Bacillus. In Hong, H.J. (ed.), Bacterial cell wall homeostasis. Methods in Molecular Biology, vol. 1440, pp. 87–98. Humana Press, New York, USA.
Heydorn, A., Nielsen, A.T., Hentzer, M., Sternberg, G., Givskov, M., Ersboll, B.K., and Molin, S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology146, 2395–2407.
Jarvis, F. and Johnson, M. 1949. A glyco-lipide produced by Pseudomonas aeruginosa. J. Am. Chem. Soc.71, 4124–4126.
Kadurugamuwa, J.L. Beveridge, T.J. 1996. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 178, 2767–2774.
Kaparakis-Liaskos, M. Ferrero, R.L. 2015. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol.15, 375–387.
Knappe, T.A., Linne, U., Zirah, S., Rebuffat, S., Xie, X., Marahiel, M.A. 2008. Isolation and structural characterization of capistruin, a lasso peptide predicted from the genome sequence of Burkholderia thailandensis E264. J. Am. Chem. Soc.130, 11446–11454.
Kondo, S., Horiuchi, Y., Hamada, M., Takeuchi, T., and Umezawa, H. 1979. A new antitumor antibiotic, bactobolin produced by Pseudomonas. Antibiot. 32, 1069–1071.
Korber, D., Lawrence, J., Hendry, M., and Caldwell, D. 1993. Analysis of spatial variability within mot+ and mot-Pseudomonas fluorescens biofilms using representative elements. Biofouling7, 339–358.
Li, Z., Clarke, A.J., Beveridge, T.J. 1998. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol.180, 5478–5483.
Li, L., Li, Y.P., Song, C.X., Xiao, M., Wang, J.L., Liu, X.S. 2014. Identification and functional characterization of a peptidogly-can recognition protein from the cotton bollworm, Helicoverpa armigera. Arch. Insect Bio chem. Physiol.86, 240–258.
Liu, X., Biswas, S., Berg, M.G., Antapli, C.M., Xie, F., Wang, Q., Tang, M.C, Tang, G.L., Zhang, L., Dreyfuss, G., et al. 2013. Ge-nomics-guided discovery of thailanstatins A, B, and C As pre-mRNA splicing inhibitors and antiproliferative agents from Burkholderia thailandensis MSMB43. J. Nat. Prod.76, 685–693.
MacDonald, K.L. Beveridge, T.J. 2002. Bactericidal effect of gentamicin-induced membrane vesicles derived from Pseudomonas aeruginosa PAOl on Gram-positive bacteria. Can. J. Microbiol.48, 810–820.
Mashburn, L.M. and Whiteley, M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature437, 422–425.
Mashburn-Warren, L., Howe, J., Garidel, P., Richter, W., Steiniger, F., Roessle, M., Brandenburg, K., and Whiteley, M. 2008a. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol.69, 491–502.
Mashburn-Warren, L, McLean, R.J., and Whiteley, M. 2008b. Gram-negative outer membrane vesicles: beyond the cell surface. Geo-biology6, 214–219.
Mashburn-Warren, L.M. and Whiteley, M. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol.61, 839–846.
Mellroth, P., Karlsson, J., and Steiner, H. 2003. A scavenger function for a Drosophila peptidoglycan recognition protein. J. Biol. Chem.278, 7059–7064.
Mihai, M.M., Holban, A.M., Giurcaneanu, C., Popa, L.G., Oanea, R.M., Lazar, V., Chifiriuc, M.C., Popa, M., Popa, M.I. 2015. Microbial biofilms: impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr. Top. Med. Chem.15, 1552–1576.
Nguyen, T., Ishida, K., Jenke-Kodama, H., Dittmann, E., Gurgui, C., Hochmuth, T., Taudien, S., Platzer, M., Hertweck, C., and Piel, J. 2008. Exploiting the mosaic structure of trans-acyltrans-ferase polyketide synthases for natural product discovery and pathway dissection. Nat. Biotechnol.26, 225–233.
Nieves, W., Asakrah, S., Qazi, O., Brown, K.A., Kurtz, J., Aucoin, D.P., McLachlan, J.B., Roy, C.J., Morici, L.A. 2011. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine29, 8381–8389.
Nieves, W., Petersen, H., Judy, B.M., Blumentritt, C.A., Russell-Lodrigue, K., Roy, C.J., Torres, A.G., Morici, L.A. 2014. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin. Vaccine Immunol.21, 747–754.
NIH. 2002. NIH guide: Research on microbial biofilms. <https://grants.nih.gov/grants/guide/pa-files/pa-03-047.html>
O’neill, J.I.M. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist.20, 1–16.
Paulino, B.N., Pessoa, M.G., Mano, M.C., Molina, G., Neri-Numa, I.A., Pastore, G.M. 2016. Current status in biotechnological production and applications of glycolipid bio surfactants. Appl. Microbiol. Biotechnol.100, 10265–10293.
Petersen, H., Nieves, W., Russell-Lodrigue, K., Roy, C.J., and Morici, L.A. 2014. Evaluation of a Burkholderia pseudomallei outer membrane vesicle vaccine in nonhuman primates. Procedia Vaccinol.8, 38–42.
Renelli, M., Matias, V., Lo, R.Y., Beveridge, T.J. 2004. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAOl and their genetic transformation potential. Microbiology150, 2161–2169.
Rosenthal, R.S. and Dziarski, R. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol.235, 253–285.
Schooling, S.R. Beveridge, T.J. 2006. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol.188, 5945–5957.
Seyedsayamdost, M.R., Chandler, J.R., Blodgett, J.A., Lima, P.S., Duerkop, B.A, Oinuma, K., Greenberg, E.P., and Clardy, J. 2010. Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Org. Lett.12, 716–719.
Sjostrom, A.E., Sandblad, L., Uhlin, B.E., Wai, S.N. 2015. Membrane vesicle-mediated release of bacterial RNA. Sci. Rep.5, 15329.
Tashiro, Y., Inagaki, A., Shimizu, M., Ichikawa, S., Takaya, N., Na-kajima-Kambe, T., Uchiyama, H., and Nomura, N. 2011. Characterization of phospholipids in membrane vesicles derived from Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem.75, 605–607.
Vial, L., Lepine, F., Milot, S., Groleau, M.C., Dekimpe, V., Woods, D.E., and Deziel, E. 2008. Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. J. Bacteriol.190, 5339–5352.
Vorregaard, M. 2008. Comstat2- a modern 3D image analysis environment for biofilms. Technical University of Denmark, DTU, DK-2800 Kgs. Lyngby, Denmark.
WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: WHO. https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/
Wood, T.L., Gong, T., Zhu, L., Miller, J., Miller, D.S., Yin, B., Wood, T.K. 2018. Rhamnolipids from Pseudomonas aeruginosa disperse the biofilms of sulfate-reducing bacteria. NPJ Biofilms Microbiomes4, 22.
Wu, Y. Seyedsayamdost, M.R. 2017. Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem. Biol.24, 1437–1444.
Acknowledgments
This work was supported by a grant to LM from the Committee on Research at Tulane University. We thank Jibao He, supervisor of the Microscopy Lab at Tulane University Coordinated Instrument Facility, for assistance with electron microscopy, and Qi Zhao, supervisor of the Organic Lab at Tulane University Coordinated Instrument Facility, for assistance with NMR spectroscopy.
Author information
Authors and Affiliations
Contributions
YW, KH, JF, JB, and LM conceived and designed the experiments. YW, JH, and CC performed the experiments. YW, CC, JB, and WW analyzed the data. YW and LM wrote the paper.
Corresponding author
Ethics declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships thatcould be construed as a potential conflict of interest.
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, Y., Hoffmann, J.P., Chou, CW. et al. Burkholderia thailandensis outer membrane vesicles exert antimicrobial activity against drug-resistant and competitor microbial species. J Microbiol. 58, 550–562 (2020). https://doi.org/10.1007/s12275-020-0028-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-020-0028-1