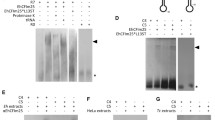
Abstract
The 25 kDa subunit of the Clevage Factor Im (CFIm25) is an essential factor for messenger RNA polyadenylation in human cells. Therefore, here we investigated whether the homologous protein of Entamoeba histolytica, the protozoan responsible for human amoebiasis, might be considered as a biochemical target for parasite control. Trophozoites were cultured with bacterial double-stranded RNA molecules targeting the EhCFIm25 gene, and inhibition of mRNA and protein expression was confirmed by RT-PCR and Western blot assays, respectively. EhCFIm25 silencing was associated with a significant acceleration of cell proliferation and cell death. Moreover, trophozoites appeared as larger and multinucleated cells. These morphological changes were accompanied by a reduced mobility, and erythrophagocytosis was significantly diminished. Lastly, the knockdown of EhCFIm25 affected the poly(A) site selection in two reporter genes and revealed that EhCFIm25 stimulates the utilization of downstream poly(A) sites in E. histolytica mRNA. Overall, our data confirm that targeting the polyadenylation process represents an interesting strategy for controlling parasites, including E. histolytica. To our best knowledge, the present study is the first to have revealed the relevance of the cleavage factor CFIm25 as a biochemical target in parasites.
Similar content being viewed by others
References
Awasthi, S. and Alwine, J.C. 2003. Association of polyadenylation cleavage factor I with U1 snRNP. RNA. 9, 1400–1409.
Barnhart, M.D., Moon, S.L., Emch, A.W., Wilusz, C.J., and Wilusz, J. 2013. Changes in cellular mRNA stability, splicing and polyadenylation through HuR protein sequestration by a cytoplasmic RNA virus. Cell Rep. 5, 909–917.
Batt, D.B., Luo, Y., and Carmichael, G.G. 1994. Polyadenylation and transcription termination in gene constructs containing multiple tandem polyadenylation signals. Nucleic Acids Res. 22, 2811–2816.
Brown, K.M. and Gilmartin, G.M. 2003. A mechanism for the regulation of pre-mRNA 3????processing by human cleavage factor Im. Mol. Cell 12, 1467–1476.
Caput, D., Beutler, B., Hartog, K., Thayer, R., Brown-Shimer, S., and Cerami, A. 1986. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc. Natl. Acad. Sci. USA 83, 1670–1674.
Colgan, D.F. and Manley, J.L. 2016. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 11, 2755–2766.
Curinha, A., Braz, S.O., Pereira-Castro, I., Cruz, A., and Moreira, A. 2014. Implications of polyadenylation in health and disease. Nucleus 5, 508–519.
De, S., Pal, D., and Ghosh, S.K. 2006. Entamoeba histolytica: computational identification of putative microRNA candidates. Exp. Parasitol. 113, 239–243.
De Chaumont, F., Dallongeville, S., Chenouard, N., Hervé, N., Pop, S., Provoost, T., Meas-Yedid, V., Pankajakshan, P., Lecomte, T., Le Montagner, Y., et al. 2012. Icy: an open bioimage informatics platform for extended reproducible research. Nat. Methods 9, 690–696.
De Vries, H., Rüegsegger, U., Hübner, W., Friedlein, A., Langen, H., and Keller, W. 2000. Human pre-mRNA cleavage factor IIm contains homologs of yeast proteins and bridges two other cleavage factors. EMBO J. 19, 5895–5904.
Derti, A., Garrett-Engele, P., Macisaac, K.D., Stevens, R.C., Sriram, S., Chen, R., Rohl, C.A., Johnson, J.M., and Babak, T. 2012. A quantitative atlas of polyadenylation in five mammals. Genome Res. 22, 1173–1183.
Diamond, L.S., Harlow, D.R., and Cunnick, C.C. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72, 431–432.
Fan, X.C. and Steitz, J.A. 1998. Overexpression of HuR, a nuclearcytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17, 3448–3460.
Fukumitsu, H., Soumiya, H., and Furukawa, S. 2012. Knockdown of pre-mRNA cleavage factor Im 25 kDa promotes neurite outgrowth. Biochem. Bioph. Res. Co. 425, 848–853.
Gilmartin, G.M. 2005. Eukaryotic mRNA 3′ processing: a common means to different ends. Genes Dev. 19, 2517–2521.
Hernández de la Cruz, O., Marchat, L.A, Guillén, N., Weber, C., López-Rosas, I., Díaz-Chávez, J. Herrera, L., Rojo-Domínguez, A., Orozco, E., and López-Camarillo, C. 2016. Multinucleation and polykaryon formation is promoted by the EhPC4 transcription factor in Entamoeba histolytica. Sci. Rep. 6, 19611.
Hernández de la Cruz, O., Muñiz-Lino, M., Guillén, N., Weber, C., Marchat, L.A, López-Rosas, I. Ruíz-García, E., Astudillo-de la Vega, H., Fuentes-Mera, L., Álvarez-Sánchez, E., et al. 2014. Proteomic profiling reveals that EhPC4 transcription factor induces cell migration through up-regulation of the 16-kDa actin-binding protein EhABP16 in Entamoeba histolytica. J. Proteomics 111, 46–58.
Hon, C.C., Weber, C., Sismeiro, O., Proux, C., Koutero, M., Deloger, M., Das, S., Agrahari, M., Dillies, M.A., Jagla, B., et al. 2013. Quantification of stochastic noise of splicing and polyadenylation in Entamoeba histolytica. Nucleic Acids Res. 41, 1936–1952.
Ingham, R.J., Colwill, K., Howard, C., Dettwiler, S., Lim, C.S.H., and Yu, J. 2005. WW domains provide a platform for the assembly of multiprotein networks. Mol. Cell. Biol. 25, 7092–7106.
Kalathur, R.K.R., Pinto, J.P., Hernández-Prieto, M.A., Machado, R.S.R., Almeida, D., Chaurasia, G., and Futschik, M.E. 2013. UniHI 7: an enhanced database for retrieval and interactive analysis of human molecular interaction networks. Nucleic Acids Res. 42, D408–D414.
Kim, S., Yamamoto, J., Chen, Y., Aida, M., Wada, T., Handa, H., and Yamaguchi, Y. 2010. Evidence that cleavage factor Im is a heterotetrameric protein complex controlling alternative polyadenylation. Genes Cells 15, 1003–1013.
Klaff, P., Riesner, D., and Steger, G. 1996. RNA structure and the regulation of gene expression. Plant Mol. Biol. 32, 89–106.
Kubo, T., Wada, T., Yamaguchi, Y., Shimizu, A., and Handa, H. 2006. Knock-down of 25 kDa subunit of cleavage factor Im in Hela cells alters alternative polyadenylation within 30-UTRs. Nucleic Acids Res. 34, 6264–6271.
López-Camarillo, C., Luna-Arias, J.P., Marchat, L.A., and Orozco, E. 2003. EhPgp5 mRNA stability is a regulatory event in the Entamoeba histolytica multidrug resistance phenotype. J. Biol. Chem. 278, 11273–11280.
López-Camarillo, C., Orozco, E., and Marchat, L.A. 2005. Entamoeba histolytica: Comparative genomics of the pre-mRNA 3′ end processing machinery. Exp. Parasitol. 110, 184–190.
Okagaki, L.H., Strain, A.K., Nielsen, J.N., Charlier, C., Baltes, N.J., Chrétien, F., Heitman, F., Dromer, F., and Nielsen, K. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 6, e1000953.
Ospina-Villa, J.D., Zamorano-Carrillo, A., López-Camarillo, C., Castañon-Sánchez, C.A., Soto-Sánchez, J., Ramírez-Moreno, M.E., and Marchat, L.A. 2015. Amino acid residues Leu135 and Tyr236 are required for RNA binding activity of CFIm25 in Entamoeba histolytica. Biochimie 115, 44–51.
Pezet-Valdez, M., Fernández-Retana, J., Ospina-Villa, J.D., Ramírez-Moreno, M.E., Orozco, E., Charcas-López, S., Soto-Sánchez, J., Mendoza-Hernández, G., López-Casamicha, M., López-Camarillo, C., et al. 2013. The 25 kDa subunit of cleavage factor Im is a RNAbinding protein that interacts with the poly(A) polymerase in Entamoeba histolytica. PLoS One 8, e67977.
Ralston, K.S. and Petri, W.A. Jr. 2011. Tissue destruction and invasion by Entamoeba histolytica. Trends Parasitol. 27, 254–263.
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675.
Sikorski, T.W., Ficarro, S.B., Holik, J., Kim, T., Rando, O.J., Marto, J.A., and Buratowski, S. 2011. Sub1 and RPA associate with RNA polymerase II at different stages of transcription. Mol. Cell. 44, 397–409.
Solis, C.F., Santi-Rocca, J., Perdomo, D., Weber, C., and Guillén, N. 2009. Use of bacterially expressed dsRNA to down regulate Entamoeba histolytica gene expression. PLoS One 4, e8424.
Takiff, H.E., Chen, S.M., and Court, D.L. 1989. Genetic analysis of the rnc operon of Escherichia coli. J. Bacteriol. 171, 2581–2590.
Tourrière, H., Chebli, K., and Tazi, J. 2002. mRNA degradation machines in eukaryotic cells. Biochimie 84, 821–837.
Vasudevan, S., Tong, Y., and Steitz, J.A. 2007. Switching from repression to activation: microRNAs can up-regulate translation. Science 318, 1931–1934.
Vinayagam, A., Stelzl, U., Foulle, R., Plassmann, S., Zenkner, M., Timm, J., Assmus, H.E., Andrade-Navarro, M.A., and Wanker, E.E. 2011. A directed protein Interaction network for investigating intracellular signal transduction. Science Signaling. 4, rs8.
Wang, S.W., Asakawa, K., Win, T.Z., Toda, T., and Norbury, C.J. 2005. Inactivation of the pre-mRNA cleavage and polyadenylation factor Pfs2 in fission yeast causes lethal cell cycle defects. Mol. Cell. Biol. 25, 2288–2296.
Yang, Q., Coseno, M., Gilmartin, G.M., and Doublié, S. 2011. Crystal structure of a human cleavage factor CFIm25/CFIm68/RNA complex provides an insight into poly(A) site recognition and RNA looping. Structure 19, 368–377.
Zamorano, A., López-Camarillo, C., Orozco, E., Weber, C., Guillén, N., and Marchat, L.A. 2008. In silico analysis of EST and genomic sequences allowed the prediction of cis-regulatory elements for Entamoeba histolytica mRNA polyadenylation. Comput. Biol. Chem. 32, 256–263.
Zhang, W., Wagner, B.J., Ehrenman, K., Schaefer, A.W., DeMaria, C.T., Crater, D., DeHaven, K., Long, L., and Brewer, G. 1993. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol. Cell. Biol. 13, 7652–7665.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Supplementary material, approximately 11.2 MB.
Supplementary material, approximately 11.2 MB.
Supplementary material, approximately 20.8 MB.
Supplementary material, approximately 19.5 MB.
Rights and permissions
About this article
Cite this article
Ospina-Villa, J.D., Guillén, N., Lopez-Camarillo, C. et al. Silencing the cleavage factor CFIm25 as a new strategy to control Entamoeba histolytica parasite. J Microbiol. 55, 783–791 (2017). https://doi.org/10.1007/s12275-017-7259-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-017-7259-9