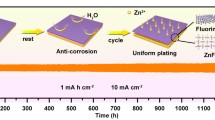
Abstract
The urgent need for highly safe and sustainable large-scale energy storage systems for residential buildings has led to research into aqueous zinc ion batteries. However, when zinc is used in aqueous zinc ion batteries, it suffers from severe irreversibility due to its low Coulombic efficiency, dendrite growth, and side reactions. To address these challenges, we take advantage of organic cation to induce trifluoromethanesulfonate decomposition to build zinc fluoride/zinc sulfide-rich solid electrolyte interphase (SEI) that not only can adapt to a high areal capacity of deposition/stripping disturbance but also adjust zinc ion deposition path to eliminate dendrite. As a result, the unique interface can promote the Zn battery to achieve excellent electrochemical performance: high levels of plating/stripping Coulombic efficiency (99.8%), stability life (6,600 h), and cumulative capacity (66,000 mAh·cm−2) at 68% zinc utilization (20 mAh·cm−2). More importantly, the SEI significantly enhances the cyclability of full battery under limited Zn, lean electrolyte, and high areal capacity cathode conditions.

Similar content being viewed by others
References
Wang, Z. X.; Sun, Z. H.; Li, J.; Shi, Y.; Sun, C. G.; An, B. G.; Cheng, H. M.; Li, F. Insights into the deposition chemistry of Li ions in nonaqueous electrolyte for stable Li anodes. Chem. Soc. Rev. 2021, 50, 3178–3210.
Dong, H.; Tutusaus, O.; Liang, Y. L.; Zhang, Y.; Lebens-Higgins, Z.; Yang, W. L.; Mohtadi, R.; Yao, Y. High-power Mg batteries enabled by heterogeneous enolization redox chemistry and weakly coordinating electrolytes. Nat. Energy 2020, 5, 1043–1050.
Wang, W. L.; Gang, Y.; Hu, Z.; Yan, Z. C.; Li, W. J.; Li, Y. C.; Gu, Q. F.; Wang, Z. X.; Chou, S. L.; Liu, H. K. et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun. 2020, 11, 980.
Ma, L. T.; Chen, S. M.; Li, N.; Liu, Z. X.; Tang, Z. J.; Zapien, J. A.; Chen, S. M.; Fan, J.; Zhi, C. Y. Hydrogen-free and dendrite-free all-solid-state Zn-ion batteries. Adv. Mater. 2020, 32, 1908121.
Choi, J. W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013.
Li, H. F.; Ma, L. T.; Han, C. P.; Wang, Z. F.; Liu, Z. X.; Tang, Z. J.; Zhi, C. Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587.
Zhang, H.; Liu, X.; Li, H. H.; Hasa, I.; Passerini, S. Challenges and strategies for high-energy aqueous electrolyte rechargeable batteries. Angew. Chem., Int. Ed. 2021, 60, 598–616.
Chen, T. T.; Wang, F. F.; Cao, S.; Bai, Y.; Zheng, S. S.; Li, W. T.; Zhang, S. T.; Hu, S. X.; Pang, H. In situ synthesis of MOF-74 family for high areal energy density of aqueous nickel-zinc batteries. Adv. Mater. 2022, 34, 2201779.
Gao, S. S.; Chen, S. S.; Liu, Q.; Zhang, S. S.; Qi, G. C.; Luo, J.; Liu, X. J. Bifunctional BiPd alloy particles anchored on carbon matrix for reversible Zn-CO2 battery. ACS Appl. Nano Mater. 2022, 5, 12387–12394.
Liu, S.; Jin, M. M.; Sun, J. Q.; Qin, Y. J.; Gao, S. S.; Chen, Y.; Zhang, S. S.; Luo, J.; Liu, X. J. Coordination environment engineering to boost electrocatalytic CO2 reduction performance by introducing boron into single-Fe-atomic catalyst. Chem. Eng. J. 2022, 437, 135294.
Liu, W. X.; Feng, J. X.; Wei, T. R.; Liu, Q.; Zhang, S. S.; Luo, Y.; Luo, J.; Liu, X. J. Active-site and interface engineering of cathode materials for aqueous Zn-gas batteries. Nano Res., in press, https://doi.org/10.1007/s12274-022-4929-7.
Yu, H.; Zeng, Y. X.; Li, N. W.; Luan, D. Y.; Yu, L.; Lou, X. W. Confining Sn nanoparticles in interconnected N-doped hollow carbon spheres as hierarchical zincophilic fibers for dendrite-free Zn metal anodes. Sci. Adv. 2022, 8, eabm5766.
Parker, J. F.; Chervin, C. N.; Pala, I. R.; Machler, M.; Burz, M. F.; Long, J. W.; Rolison, D. R. Rechargeable nickel-3D zinc batteries: An energy-dense, safer alternative to nickel-zinc. Science 2017, 356, 415–418.
Zampardi, G.; La Mantia, F. Open challenges and good experimental practices in the research field of aqueous Zn-ion batteries. Nat. Commun. 2022, 13, 687.
Liu, Z. X.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X. L.; Huang, Z. D.; Zhi, C. Y. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232.
Lei, Z.; Tan, Y. Y.; Zhang, Z. Y.; Wu, W.; Cheng, N. C.; Chen, R. Z.; Mu, S. C.; Sun, X. L. Defects enriched hollow porous Co-N-doped carbons embedded with ultrafine CoFe/Co nanoparticles as bifunctional oxygen electrocatalyst for rechargeable flexible solid zinc-air batteries. Nano Res. 2020, 14, 868–878.
Shan, Y. Y.; Li, Y.; Pang, H. Applications of tin sulfide-based materials in lithium-ion batteries and sodium-ion batteries. Adv. Funct. Mater. 2020, 30, 2001298.
Chen, T. T.; Bai, Y.; Xiao, X.; Pang, H. Exposing (001) crystal facet on the single crystalline β-Ni(OH)2 quasi-nanocubes for aqueous Ni-Zn batteries. Chem. Eng. J. 2021, 413, 127523.
Wang, C.; Li, J.; Zhou, Z.; Pan, Y. Q.; Yu, Z. X.; Pei, Z. X.; Zhao, S. L.; Wei, L.; Chen, Y. Rechargeable zinc-air batteries with neutral electrolytes: Recent advances, challenges, and prospects. EnergyChem 2021, 3, 100055.
He, B.; Zhang, Q. C.; Man, P.; Zhou, Z. Y.; Li, C. W.; Li, Q. L.; Xie, L. Y.; Wang, X. N.; Pang, H.; Yao, Y. G. Self-sacrificed synthesis of conductive vanadium-based metal-organic framework nanowire-bundle arrays as binder-free cathodes for high-rate and high-energy-density wearable Zn-ion batteries. Nano Energy 2019, 64, 103935.
Tian, Y. D.; Chen, S.; He, Y. L.; Chen, Q. W.; Zhang, L. L.; Zhang, J. T. A highly reversible dendrite-free Zn anode via spontaneous galvanic replacement reaction for advanced zinc-iodine batteries. Nano Res. Energy 2022, 1, e9120025.
Liu, S.; Wang, L.; Yang, H.; Gao, S. S.; Liu, Y. F.; Zhang, S. S.; Chen, Y.; Liu, X. J.; Luo, J. Nitrogen-doped carbon polyhedrons confined Fe-P nanocrystals as high-efficiency bifunctional catalysts for aqueous Zn-CO2 batteries. Small 2022, 18, 2104965.
Gao, S. S.; Wei, T. R.; Sun, J. Q.; Liu, Q.; Ma, D.; Liu, W. X.; Zhang, S. S.; Luo, J.; Liu, X. J. Atomically dispersed metal-based catalysts for Zn-CO2 batteries. Small Struct., in press, https://doi.org/10.1002/sstr.202200086.
Gu, J. W.; Peng, Y.; Zhou, T.; Ma, J.; Pang, H.; Yamauchi, Y. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1, e9120009.
Yang, Q.; Li, Q.; Liu, Z. X.; Wang, D. H.; Guo, Y.; Li, X. L.; Tang, Y. C.; Li, H. F.; Dong, B. B.; Zhi, C. Y. Dendrites in Zn-based batteries. Adv. Mater. 2020, 32, 2001854.
Hao, J. N.; Li, X. L.; Zeng, X. H.; Li, D.; Mao, J. F.; Guo, Z. P. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ. Sci. 2020, 13, 3917–3949.
Zeng, Y. X.; Lu, X. F.; Zhang, S. L.; Luan, D. Y.; Li, S.; Lou, X. W. Construction of Co-Mn Prussian blue analog hollow spheres for efficient aqueous Zn-ion batteries. Angew. Chem., Int. Ed. 2021, 60, 22189–22194.
Du, Y. H.; Wang, X. Y.; Sun, J. C. Tunable oxygen vacancy concentration in vanadium oxide as mass-produced cathode for aqueous zinc-ion batteries. Nano Res. 2021, 14, 754–761.
Chen, X. H.; Ruan, P. C.; Wu, X. W.; Liang, S. Q.; Zhou, J. Crystal structures, reaction mechanisms, and optimization strategies of MnO2 cathode for aqueous rechargeable zinc batteries. Acta Phys. Chim. Sin. 2022, 38, 2111003.
Ruan, P. C.; Xu, X. L.; Zheng, D.; Chen, X. H.; Yin, X. Y.; Liang, S. Q.; Wu, X. W.; Shi, W. H.; Cao, X. H.; Zhou, J. Promoting reversible dissolution/deposition of MnO2 for high-energy-density zinc batteries via enhancing cut-off voltage. ChemSusChem 2022, 15, e202201118.
Shin, J.; Lee, J.; Park, Y.; Choi, J. W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044.
Cao, L. S.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C. Y.; Chen, L.; Vatamanu, J.; Hu, E. Y.; Hourwitz, M. J. et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat. Nanotechnol. 2021, 16, 902–910.
Bayer, M.; Overhoff, G. M.; Gui, A. L.; Winter, M.; Bieker, P.; Schulze, S. Influence of water content on the surface morphology of zinc deposited from EMImOTf/water mixtures. J. Electrochem. Soc. 2019, 166, A909–A914.
Higashi, S.; Lee, S. W.; Lee, J. S.; Takechi, K.; Cui, Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat. Commun. 2016, 7, 11801.
Wang, F.; Borodin, O.; Gao, T.; Fan, X. L.; Sun, W.; Han, F. D.; Faraone, A.; Dura, J. A.; Xu, K.; Wang, C. S. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549.
Chu, Y. Z.; Zhang, S.; Wu, S.; Hu, Z. L.; Cui, G. L.; Luo, J. Y. In situ built interphase with high interface energy and fast kinetics for high performance Zn metal anodes. Energy Environ. Sci. 2021, 14, 3609–3620.
Qiu, H. Y.; Du, X. F.; Zhao, J. W.; Wang, Y. T.; Ju, J. W.; Chen, Z.; Hu, Z. L.; Yan, D. P.; Zhou, X. H.; Cui, G. L. Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation. Nat. Commun. 2019, 10, 5374.
Zhang, Q.; Ma, Y. L.; Lu, Y.; Li, L.; Wan, F.; Zhang, K.; Chen, J. Modulating electrolyte structure for ultralow temperature aqueous zinc batteries. Nat. Commun. 2020, 11, 4463.
Chen, Z.; Tang, Y.; Du, X. F.; Chen, B. B.; Lu, G. L.; Han, X. Q.; Zhang, Y. J.; Yang, W. H.; Han, P. X.; Zhao, J. W. et al. Anion solvation reconfiguration enables high-voltage carbonate electrolytes for stable Zn/graphite cells. Angew. Chem., Int. Ed. 2020, 59, 21769–21777.
Ma, L.; Pollard, T. P.; Zhang, Y.; Schroeder, M. A.; Ding, M. S.; Cresce, A. V.; Sun, R. M.; Baker, D. R.; Helms, B. A.; Maginn, E. J. et al. Functionalized phosphonium cations enable zinc metal reversibility in aqueous electrolytes. Angew. Chem., Int. Ed. 2021, 60, 12438–12445.
Lv, Y. Q.; Zhao, M.; Du, Y. D.; Kang, Y.; Xiao, Y.; Chen, S. M. Engineering a self-adaptive electric double layer on both electrodes for high-performance zinc metal batteries. Energy Environ. Sci. 2022, 15, 4748–4760.
Cao, F. Q.; Wu, B. H.; Li, T. Y.; Sun, S. T.; Jiao, Y. C.; Wu, P. Y. Mechanoadaptive morphing gel electrolyte enables flexible and fast-charging Zn-ion batteries with outstanding dendrite suppression performance. Nano Res. 2022, 15, 2030–2039.
Zhao, Z. M.; Zhao, J. W.; Hu, Z. L.; Li, J. D.; Li, J. J.; Zhang, Y. J.; Wang, C.; Cui, G. L. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949.
Zhao, Z. D.; Wang, R.; Peng, C. X.; Chen, W. J.; Wu, T. Q.; Hu, B.; Weng, W. J.; Yao, Y.; Zeng, J. X.; Chen, Z. H. et al. Horizontally arranged zinc platelet electrodeposits modulated by fluorinated covalent organic framework film for high-rate and durable aqueous zinc ion batteries. Nat. Commun. 2021, 12, 6606.
Zhang, L.; Zhang, B.; Zhang, T.; Li, T.; Shi, T. F.; Li, W.; Shen, T.; Huang, X. X.; Xu, J. J.; Zhang, X. G. et al. Eliminating dendrites and side reactions via a multifunctional ZnSe protective layer toward advanced aqueous Zn metal batteries. Adv. Funct. Mater. 2021, 31, 2100186.
Hao, J. N.; Li, B.; Li, X. L.; Zeng, X. H.; Zhang, S. L.; Yang, F. H.; Liu, S. L.; Li, D.; Wu, C.; Guo, Z. P. An in-depth study of Zn metal surface chemistry for advanced aqueous Zn-ion batteries. Adv. Mater. 2020, 32, 2003021.
Tian, H. J.; Li, Z.; Feng, G. X.; Yang, Z. Z.; Fox, D.; Wang, M. Y.; Zhou, H.; Zhai, L.; Kushima, A.; Du, Y. G. et al. Stable, high-performance, dendrite-free, seawater-based aqueous batteries. Nat. Commun. 2021, 12, 237.
Wang, S. B.; Ran, Q.; Yao, R. Q.; Shi, H.; Wen, Z.; Zhao, M.; Lang, X. Y.; Jiang, Q. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat. Commun. 2020, 11, 1634.
Fayette, M.; Chang, H. J.; Li, X. L.; Reed, D. High-performance InZn alloy anodes toward practical aqueous zinc batteries. ACS Energy Lett. 2022, 7, 1888–1895.
Li, Q. Y.; Chen, Y. J.; Wang, H.; Yu, H. M.; Wei, W. F.; Ji, X. B.; Qu, B. H.; Chen, L. B. Advances in the structure and composition design of zinc anodes for high performance zinc ion batteries. Sustainable Energy Fuels 2022, 6, 3501–3515.
Zhang, Q.; Ma, Y. L.; Lu, Y.; Zhou, X. Z.; Lin, L.; Li, L.; Yan, Z. H.; Zhao, Q.; Zhang, K.; Chen, J. Designing anion-type water-free Zn2+ solvation structure for robust Zn metal anode. Angew. Chem., Int. Ed. 2021, 60, 23357–23364.
Naveed, A.; Yang, H. J.; Yang, J.; Nuli, Y.; Wang, J. L. Highly reversible and rechargeable safe Zn batteries based on a triethyl phosphate electrolyte. Angew. Chem., Int. Ed. 2019, 58, 2760–2764.
Naveed, A.; Yang, H. J.; Shao, Y. Y.; Yang, J.; Yanna, N.; Liu, J.; Shi, S. Q.; Zhang, L. W.; Ye, A. J.; He, B. et al. A highly reversible Zn anode with intrinsically safe organic electrolyte for long-cycle-life batteries. Adv. Mater. 2019, 31, 1900668.
Ming, F. W.; Zhu, Y. P.; Huang, G.; Emwas, A. H.; Liang, H. F.; Cui, Y.; Alshareef, H. N. Co-solvent electrolyte engineering for stable anode-free zinc metal batteries. J. Am. Chem. Soc. 2022, 144, 7160–7170.
Ma, Y. L.; Zhang, Q.; Liu, L. J.; Li, Y. X.; Li, H. X.; Yan, Z. H.; Chen, J. N, N-dimethylformamide tailors solvent effect to boost Zn anode reversibility in aqueous electrolyte. Natl. Sci. Rev., in press, https://doi.org/10.1093/nsr/nwac051.
Bayaguud, A.; Luo, X.; Fu, Y. P.; Zhu, C. B. Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries. ACS Energy Lett. 2020, 5, 3012–3020.
Liu, Y. J.; Tao, X. Y.; Wang, Y.; Jiang, C.; Ma, C.; Sheng, O. W.; Lu, G. X.; Lou, X. W. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 2022, 375, 739–745.
Chen, J.; Fan, X. L.; Li, Q.; Yang, H. B.; Khoshi, M. R.; Xu, Y. B.; Hwang, S.; Chen, L.; Ji, X.; Yang, C. Y. et al. Electrolyte design for LiF-rich solid-electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 2020, 5, 386–397.
Chen, J. Z.; Zhou, W. J.; Quan, Y. H.; Liu, B.; Yang, M.; Chen, M. F.; Han, X.; Xu, X. W.; Zhang, P. X.; Shi, S. Q. Lonic liquid additive enabling anti-freezing aqueous electrolyte and dendrite-free Zn metal electrode with organic/inorganic hybrid solid electrolyte interphase layer. Energy Storage Mater. 2022, 53, 629–637.
Liu, Z.; El Abedin, S. Z.; Endres, F. Electrodeposition of zinc films from ionic liquids and ionic liquid/water mixtures. Electrochim. Acta 2013, 89, 635–643.
Liu, Z.; El Abedin, S. Z.; Borisenko, N.; Endres, F. Influence of an additive on zinc electrodeposition in the ionic liquid 1-ethyl-3-methylimidazolium trifluoromethylsulfonate. ChemElectroChem 2015, 2, 1159–1163.
Ma, L.; Vatamanu, J.; Hahn, N. T.; Pollard, T. P.; Borodin, O.; Petkov, V.; Schroeder, M. A.; Ren, Y.; Ding, M. S.; Luo, C. et al. Highly reversible Zn metal anode enabled by sustainable hydroxyl chemistry. Proc. Natl. Acad. Sci. USA 2022, 119, e2121138119.
Shan, L. T.; Zhou, J.; Zhang, W. Y.; Xia, C. T.; Guo, S.; Ma, X. M.; Fang, G. Z.; Wu, X. W.; Liang, S. Q. Highly reversible phase transition endows V6O13 with enhanced performance as aqueous zinc-ion battery cathode. Energy Technol. 2019, 7, 1900022.
Ghanbari, K.; Mousavi, M. F.; Shamsipur, M. Preparation of polyaniline nanofibers and their use as a cathode of aqueous rechargeable batteries. Electrochim. Acta 2006, 52, 1514–1522.
Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J. Chem. Phys. 2002, 117, 43–54.
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J. Phys. Chem. B 2009, 113, 4538–4543.
Zhao, Y.; Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241.
Zhao, Y.; Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167.
Krishnan, R.; Binkley, J. S.; Seeger, R.; Pople, J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654.
McLean, A.; Chandler, G. S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11−18. J. Chem. Phys. 1980, 72, 5639–5648.
Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141.
Igel-Mann, G.; Stoll, H.; Preuss, H. Pseudopotentials for main group elements (IIIa through VIIa). Mol. Phys. 1988, 65, 1321–1328.
Bauernschmitt, R.; Ahlrichs, R. Stability analysis for solutions of the closed shell Kohn—Sham equation. J. Chem. Phys. 1996, 104, 9047–9052.
Zhao, K.; Fan, G. L.; Liu, J. D.; Liu, F. M.; Li, J. H.; Zhou, X. Z.; Ni, Y. X.; Yu, M.; Zhang, Y. M.; Su, H. et al. Boosting the kinetics and stability of Zn anodes in aqueous electrolytes with supramolecular cyclodextrin additives. J. Am. Chem. Soc. 2022, 144, 11129–11137.
Zhao, K.; Liu, F. M.; Fan, G. L.; Liu, J. D.; Yu, M.; Yan, Z. H.; Zhang, N.; Cheng, F. Y. Stabilizing zinc electrodes with a vanillin additive in mild aqueous electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 47650–47658.
Li, Q.; Chen, A.; Wang, D. H.; Pei, Z. X.; Zhi, C. Y. “Soft shorts” hidden in zinc metal anode research. Joule 2022, 6, 273–279.
Xu, W. N.; Zhao, K. N.; Huo, W. C.; Wang, Y. Z.; Yao, G.; Gu, X.; Cheng, H. W.; Mai, L. Q.; Hu, C. G.; Wang, X. D. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019, 62, 275–281.
Wang, Z. Q.; Hu, J. T.; Han, L.; Wang, Z. J.; Wang, H. B.; Zhao, Q. H.; Liu, J. J.; Pan, F. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 2019, 56, 92–99.
Zhao, J. W.; Zhang, J.; Yang, W. H.; Chen, B. B.; Zhao, Z. M.; Qiu, H. Y.; Dong, S. M.; Zhou, X. H.; Cui, G. L.; Chen, L. Q. “Water-in-deep eutectic solvent” electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 2019, 57, 625–634.
Zhang, C.; Holoubek, J.; Wu, X. Y.; Daniyar, A.; Zhu, L. D.; Chen, C.; Leonard, D. P.; Rodríguez-Pérez, I. A.; Jiang, J. X.; Fang, C. et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. (Camb.) 2018, 54, 14097–14099.
Cui, Y. H.; Zhao, Q. H.; Wu, X. J.; Wang, Z. J.; Qin, R. Z.; Wang, Y. T.; Liu, M. Q.; Song, Y. L.; Qian, G. Y.; Song, Z. B. et al. Quasi-solid single Zn-ion conductor with high conductivity enabling dendrite-free Zn metal anode. Energy Storage Mater. 2020, 27, 1–8.
Zeng, X. H.; Liu, J. T.; Mao, J. F.; Hao, J. N.; Wang, Z. J.; Zhou, S.; Ling, C. D.; Guo, Z. P. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy Mater. 2020, 10, 1904163.
Zhu, Y. P.; Yin, J.; Zheng, X. L.; Emwas, A. H.; Lei, Y. J.; Mohammed, O. F.; Cui, Y.; Alshareef, H. N. Concentrated dual-cation electrolyte strategy for aqueous zinc-ion batteries. Energy Environ. Sci. 2021, 14, 4463–4473.
Sun, P.; Ma, L.; Zhou, W. H.; Qiu, M. J.; Wang, Z. L.; Chao, D. L.; Mai, W. J. Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries achieved by a low-cost glucose additive. Angew. Chem., Int. Ed. 2021, 60, 18247–18255.
Huang, C.; Zhao, X.; Liu, S.; Hao, Y. S.; Tang, Q. L.; Hu, A. P.; Liu, Z. X.; Chen, X. H. Stabilizing zinc anodes by regulating the electrical double layer with saccharin anions. Adv. Mater. 2021, 33, 2100445.
Zhang, S. J.; Hao, J. N.; Luo, D.; Zhang, P. F.; Zhang, B. K.; Davey, K.; Lin, Z.; Qiao, S. Z. Dual-function electrolyte additive for highly reversible Zn anode. Adv. Energy Mater. 2021, 11, 2102010.
Qin, R. Z.; Wang, Y. T.; Zhang, M. Z.; Wang, Y.; Ding, S. X.; Song, A. Y.; Yi, H. C.; Yang, L. Y.; Song, Y. L.; Cui, Y. H. et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021, 80, 105478.
Fan, L.; Ma, R. F.; Zhang, Q. F.; Jia, X. X.; Lu, B. A. Graphite anode for a potassium-ion battery with unprecedented performance. Angew. Chem., Int. Ed. 2019, 58, 10500–10505.
Yang, Y.; Liu, C. Y.; Lv, Z. H.; Yang, H.; Zhang, Y. F.; Ye, M. H.; Chen, L. B.; Zhao, J. B.; Li, C. C. Synergistic manipulation of Zn2+ ion flux and desolvation effect enabled by anodic growth of a 3D ZnF2 matrix for long-lifespan and dendrite-free Zn metal anodes. Adv. Mater. 2021, 33, 2007388.
Li, F.; He, J.; Liu, J. D.; Wu, M. G.; Hou, Y. Y.; Wang, H. P.; Qi, S. H.; Liu, Q. H.; Hu, J. W.; Ma, J. M. Gradient solid electrolyte interphase and lithium-ion solvation regulated by bisfluoroacetamide for stable lithium metal batteries. Angew. Chem., Int. Ed. 2021, 60, 6600–6608.
Bouibes, A.; Takenaka, N.; Kubota, K.; Komaba, S.; Nagaoka, M. Development of advanced electrolytes in Na-ion batteries: Application of the red moon method for molecular structure design of the SEI layer. RSC Adv. 2022, 12, 971–984.
Xiao, N.; Gourdin, G.; Wu, Y. Y. Simultaneous stabilization of potassium metal and superoxide in K-O2 batteries on the basis of electrolyte reactivity. Angew. Chem., Int. Ed. 2018, 57, 10864–10867.
Chen, Y.; Cao, Y. Y.; Shi, Y.; Xue, Z. M.; Mu, T. C. Quantitative research on the vaporization and decomposition of [Tf2N] by thermogravimetric analysis-mass spectrometry. Ind. Eng. Chem. Res. 2012, 51, 7418–7427.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 2227912129) and Joint Fund of Scientific and Technological Research and Development Program of Henan Province (No. 222301420009). The Center of Advanced Analysis & Gene Sequencing of Zhengzhou University was thanked for Cryo-TEM testing.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Ge, J., Zhang, Y., Xie, Z. et al. Tailored ZnF2/ZnS-rich interphase for reversible aqueous Zn batteries. Nano Res. 16, 4996–5005 (2023). https://doi.org/10.1007/s12274-022-5325-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5325-z