Abstract
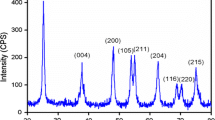
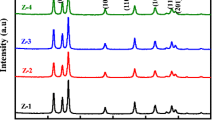
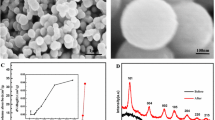
A facile inside-out Ostwald ripening route to the morphology-controlled preparation of TiO2 microspheres is developed. Here, TiO2 hollow microspheres (HM) and solid microspheres (SM) are prepared by adjusting the volume ratio of isopropanol (IPA) to acetylacetone (Acac) in the solvothermal process. During the formation process of HM, precipitation of solid cores, subsequent deposition of outer shells on the surface of cores, and simultaneous core dissolution and shell recrystallization are observed, which validate the inside-out Ostwald ripening mechanism. Design and optimization of the properties (pore size, surface area, and trap state) of TiO2 microspheres are vital to the high performance of dyesensitized solar cells (DSSCs). The optimized TiO2 microspheres (rHM and rSM) obtained by post-processing on recrystallization, possess large pore sizes, high surface areas and reduced trap states (Ti3+ and oxygen vacancy), and are thus ideal materials for photovoltaic devices. The power conversion efficiency of DSSCs fabricated using rHM photoanode is 11.22%, which is significantly improved compared with the 10.54% efficiency of the rSM-based DSSC. Our work provides a strategy for synthesizing TiO2 microspheres that simultaneously accommodate different physical properties, in terms of surface area, crystallinity, morphology, and mesoporosity.

Similar content being viewed by others
References
O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740.
Grätzel, M. All surface and no bulk. Nature 1991, 349, 740–741.
Sauvage, F.; Chen, D. H.; Comte, P.; Huang, F. Z.; Heiniger, L. P.; Cheng, Y. B.; Caruso, R. A.; Graetzel, M. Dyesensitized solar cells employing a single film of mesoporous TiO2 beads achieve power conversion efficiencies over 10%. ACS Nano 2010, 4, 4420–4425.
Hwang, S. H.; Yun, J.; Jang, J. Multi-shell porous TiO2 hollow nanoparticles for enhanced light harvesting in dyesensitized solar cells. Adv. Funct. Mater. 2014, 24, 7619–7626.
Lou, X. W.; Archer, L. A.; Yang, Z. C. Hollow micro-/nanostructures: Synthesis and applications. Adv. Mater. 2008, 20, 3987–4019.
Wu, X.; Lu, G. Q.; Wang, L. Z. Shell-in-shell TiO2 hollow spheres synthesized by one-pot hydrothermal method for dye-sensitized solar cell application. Energy Environ. Sci. 2011, 4, 3565–3572.
Xi, J. T.; Zhang, Q. F.; Xie, S. H.; Yodyingyong, S.; Park, K.; Sun, Y. M.; Li, J. Y.; Cao, G. Z. Fabrication of TiO2 aggregates by electrospraying and their application in dyesensitized solar cells. Nanosci. Nanotech. Let. 2011, 3, 690–696.
Li, X. X.; Xiong, Y. J.; Li, Z. Q.; Xie, Y. Large-scale fabrication of TiO2 hierarchical hollow spheres. Inorg. Chem. 2006, 45, 3493–3495.
Li, W. J.; Coppens, M. O. Synthesis and characterization of stable hollow Ti-silica microspheres with a mesoporous shell. Chem. Mater. 2005, 17, 2241–2246.
Yang, H. G.; Zeng, H. C. Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. J. Phys. Chem. B 2004, 108, 3492–3495.
Yin, Y. D.; Rioux, R. M.; Erdonmez, C. K.; Hughes, S.; Somorjai, G. A.; Alivisatos, A. P. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 2004, 304, 711–714.
Oh, M. H.; Yu, T.; Yu, S. H.; Lim, B.; Ko, K. T.; Willinger, M. G.; Seo, D. H.; Kim, B. H.; Cho, M. G.; Park, J. H. et al. Galvanic replacement reactions in metal oxide nanocrystals. Science 2013, 340, 964–968.
Hu, L. H.; Dai, S. Y.; Weng, J.; Xiao, S. F.; Sui, Y. F.; Huang, Y.; Chen, S. H.; Kong, F. T.; Pan, X.; Liang, L. Y. et al. Microstructure design of nanoporous TiO2 photoelectrodes for dye-sensitized solar cell modules. J. Phys. Chem. B 2007, 111, 358–362.
Ding, Y.; Mo, L.-E.; Tao, L.; Ma, Y.-M.; Hu, L.-H.; Huang, Y.; Fang, X.-Q.; Yao, J.-X.; Xi, X.-W.; Dai, S.-Y. TiO2 nanocrystalline layer as a bridge linking TiO2 sub-microspheres layer and substrates for high-efficiency dye-sensitized solar cells. J. Power Sources 2014, 272, 1046–1052.
Liu, B.; Liu, L.-M.; Lang, X.-F.; Wang, H.-Y.; Lou, X. W.; Aydil, E. S. Doping high-surface-area mesoporous TiO2 microspheres with carbonate for visible light hydrogen production. Energy Environ. Sci. 2014, 7, 2592–2597.
Garnweitner, G.; Antonietti, M.; Niederberger, M. Nonaqueous synthesis of crystalline anatase nanoparticles in simple ketones and aldehydes as oxygen-supplying agents. Chem. Commun. 2005, 397–399.
Yu, J. G.; Liu, W.; Yu, H. G. A one-pot approach to hierarchically nanoporous titania hollow microspheres with high photocatalytic activity. Cryst. Growth Des. 2008, 8, 930–934.
Liu, B.; Zeng, H. C. Symmetric and asymmetric Ostwald ripening in the fabrication of homogeneous core-shell semiconductors. Small 2005, 1, 566–571.
Zeng, H. C. Ostwald ripening: A synthetic approach for hollow nanomaterials. Curr. Nanosci. 2007, 3, 177–181.
Lou, X. W.; Wang, Y.; Yuan, C. L.; Lee, J. Y.; Archer, L. A. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329.
Wang, Y.; Zhu, Q. S.; Zhang, H. G. Fabrication of β-Ni(OH)2 and NiO hollow spheres by a facile template-free process. Chem. Commun. 2005, 5231–5233.
Zhao, Y. B.; Pan, F.; Li, H.; Zhao, D. X.; Liu, L.; Xu, G. Q.; Chen, W. Uniform mesoporous anatase–brookite biphase TiO2 hollow spheres with high crystallinity via Ostwald ripening. J. Phys. Chem. C 2013, 117, 21718–21723.
Widoniak, J.; Eiden-Assmann, S.; Maret, G. Synthesis and characterisation of monodisperse zirconia particles. Eur. J. Inorg. Chem. 2005, 3149–3155.
Wu, W.; Zhang, S. F.; Zhou, J.; Xiao, X. H.; Ren, F.; Jiang, C. Z. Controlled synthesis of monodisperse sub-100 nm hollow SnO2 nanospheres: A template- and surfactant-free solution-phase route, the growth mechanism, optical properties, and application as a photocatalyst. Chem.—Eur. J. 2011, 17, 9708–9719.
Peng, Z. A.; Peng, X. G. Mechanisms of the shape evolution of CdSe nanocrystals. J. Am. Chem. Soc. 2001, 123, 1389–1395.
Sugimoto, T.; Kojima, T. Formation mechanism of amorphous TiO2 spheres in organic solvents. 1. Roles of ammonia. J. Phys. Chem. C 2008, 112, 18760–18771.
Ding, Y.; Zhou, L.; Mo, L. E.; Jiang, L.; Hu, L. H.; Li, Z. Q.; Chen, S. H.; Dai, S. Y. TiO2 microspheres with controllable surface area and porosity for enhanced light harvesting and electrolyte diffusion in dye-sensitized solar cells. Adv. Funct. Mater. 2015, 25, 5946–5953.
Yang, H. G.; Sun, C. H.; Qiao, S. Z.; Zou, J.; Liu, G.; Smith, S. C.; Cheng, H. M.; Lu, G. Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641.
Shang, S. Q; Jiao, X. L.; Chen, D. R. Template-free fabrication of TiO2 hollow spheres and their photocatalytic properties. ACS Appl. Mater. Interfaces 2012, 4, 860–865.
Pan, J. H.; Wang, X. Z.; Huang, Q. Z.; Shen, C.; Koh, Z. Y.; Wang, Q.; Engel, A.; Bahnemann, D. W. Large-scale synthesis of urchin-like mesoporous TiO2 hollow spheres by targeted etching and their photoelectrochemical properties. Adv. Funct. Mater. 2014, 24, 95–104.
Wang, S. B.; Pan, L.; Song, J. J.; Mi, W. B.; Zou, J. J.; Wang, L.; Zhang, X. W. Titanium-defected undoped anatase TiO2 with p-type conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 2015, 137, 2975–2983.
Singh, J.; Gusain, A.; Saxena, V.; Chauhan, A. K.; Veerender, P.; Koiry, S. P.; Jha, P.; Jain, A.; Aswal, D. K.; Gupta, S. K. XPS, UV–Vis, FTIR, and EXAFS studies to investigate the binding mechanism of N719 dye onto oxalic acid treated TiO2 and its implication on photovoltaic properties. J. Phys. Chem. C 2013, 117, 21096–21104.
Pan, S. S.; Lu, W.; Zhao, Y. H.; Tong, W.; Li, M.; Jin, L. M.; Choi, J. Y.; Qi, F.; Chen, S. G.; Fei, L. F.; Yu, S. F. Selfdoped rutile titania with high performance for direct and ultrafast assay of H2O2. ACS Appl. Mater. Interfaces 2013, 5, 12784–12788.
Kumar, C. P.; Gopal, N. O.; Wang, T. C.; Wong, M. S.; Ke, S. C. EPR investigation of TiO2 nanoparticles with temperature-dependent properties. J. Phys. Chem. B 2006, 110, 5223–5229.
Ke, S. C.; Wang, T. C.; Wong, M. S.; Gopal, N. O. Low temperature kinetics and energetics of the electron and hole traps in irradiated TiO2 nanoparticles as revealed by EPR spectroscopy. J. Phys. Chem. B 2006, 110, 11628–11634.
Ferber, J.; Luther, J. Computer simulations of light scattering and absorption in dye-sensitized solar cells. Sol. Energ. Mat. Sol. C. 1998, 54, 265–275.
Zhang, H. M.; Yu, H.; Han, Y. H.; Liu, P. R.; Zhang, S. Q.; Wang, P.; Cheng, Y. B.; Zhao, H. J. Rutile TiO2 microspheres with exposed nano-acicular single crystals for dye-sensitized solar cells. Nano Res. 2011, 4, 938–947.
Zhang, Q. F.; Myers, D.; Lan, J. L.; Jenekhe, S. A.; Cao, G. Z. Applications of light scattering in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 14982–14998.
Heiniger, L.-P.; Giordano, F.; Moehl, T.; Grätzel, M. Mesoporous TiO2 beads offer improved mass transport for cobalt-based redox couples leading to high efficiency dyesensitized solar cells. Adv. Energy Mater. 2014, 4, 1400168.
Kang, T. S.; Smith, A. P.; Taylor, B. E.; Durstock, M. F. Fabrication of highly-ordered TiO2 nanotube arrays and their use in dye-sensitized solar cells. Nano Lett. 2009, 9, 601–606.
Zhang, W. J.; Xie, Y.; Xiong, D. H.; Zeng, X. W.; Li, Z. H.; Wang, M. K.; Cheng, Y. B.; Chen, W.; Yan, K. Y.; Yang, S. H. TiO2 nanorods: A facile size- and shape-tunable synthesis and effective improvement of charge collection kinetics for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 9698–9704.
Li, C. H.; Koenigsmann, C.; Ding, W. D.; Rudshteyn, B.; Yang, K. R.; Regan, K. P.; Konezny, S. J.; Batista, V. S.; Brudvig, G. W.; Schmuttenmaer, C. A. et al. Facet-dependent photoelectrochemical performance of TiO2 nanostructures: An experimental and computational study. J. Am. Chem. Soc. 2015, 137, 1520–1529.
Wu, X.; Chen, Z. G.; Lu, G. Q.; Wang, L. Z. Nanosized anatase TiO2 single crystals with tunable exposed (001) facets for enhanced energy conversion efficiency of dye-sensitized solar cells. Adv. Funct. Mater. 2011, 21, 4167–4172.
Chen, D. H.; Huang, F. Z.; Cheng, Y.-B.; Caruso, R. A. Mesoporous anatase TiO2 beads with high surface areas and controllable pore sizes: A superior candidate for highperformance dye-sensitized solar cells. Adv. Mater. 2009, 21, 2206–2210.
Oekermann, T.; Zhang, D. S.; Yoshida, T.; Minoura, H. Electron transport and back reaction in nanocrystalline TiO2 films prepared by hydrothermal crystallization. J. Phys. Chem. B 2004, 108, 2227–2235.
Ding, Y.; Ma, Y. M.; Tao, L.; Hu, L. H.; Li, G.; Jiang, L.; Li, Z. Q.; Mo, L. E.; Yao, J. X.; Dai, S. Y. Continuous electron transport pathways constructed in TiO2 sub-microsphere films for high-performance dye-sensitized solar cells. RSC Adv. 2015, 5, 17493–17500.
Thapa, A.; Zai, J. T.; Elbohy, H.; Poudel, P.; Adhikari, N.; Qian, X. F.; Qiao, Q. Q. TiO2 coated urchin-like SnO2 microspheres for efficient dye-sensitized solar cells. Nano Res. 2014, 7, 1154–1163.
Author information
Authors and Affiliations
Corresponding authors
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12274-016-1336-y.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ding, Y., Xia, X., Chen, W. et al. Inside-out Ostwald ripening: A facile process towards synthesizing anatase TiO2 microspheres for high-efficiency dye-sensitized solar cells. Nano Res. 9, 1891–1903 (2016). https://doi.org/10.1007/s12274-016-1081-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1081-2