Abstract
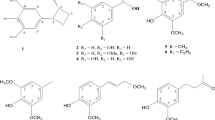
A new alkenylphenol, bavinol A (1), together with six known compounds (2–7) were isolated from the aerial parts of Piper bavinum (Piperaceae). The chemical structures of these compounds were determined by spectroscopic analyses including 2D NMR spectroscopy. The anti-Alzheimer effects of compounds 1–7 were evaluated from acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity assays. Bavinol A (1), ampelopsin (3), and violanthin (4) exhibited AChE inhibitory activities with IC50 values of 29.80, 59.47 and 79.80 μM. Compound 1 also showed the most potent BChE inhibitory activity with an IC50 value of 19.25 μM.


Similar content being viewed by others
References
Barrett, B. 1994. Medicinal plants of Nicaragua’s Atlantic Coast. Economic Botany 48: 8–20.
Chaurasia, S., and R. Saxena. 2012. Antibacterial activity of four different varieties of green beans. Research Journal of Pharmaceutical, Biological and Chemical Sciences 3: 70–74.
Cummings, J.L. 2000. Cholinesterase inhibitors: Expanding applications. Lancet 356: 2024–2025.
Doss, A., and M. Pugualenthi. 2012. Evaluation of antioxydant activity and phytochemical screening of Malus domestica Borkh (apple) and Phaseolus vulgaris L. (green beans). Journal of Pharmaceutical and Scientific Innovation 3: 1–4.
Ellman, G.L., K.D. Courtney, V.J. Andres, and R.M. Featherstone. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7: 88–95.
Fan, P., A.E. Hay, A. Marston, and K. Hostettmann. 2008. Acetylcholinesterase-inhibitory activity of linarin from Buddleja davidii, structure-activity relationships of related flavonoids, and chemical investigation of Buddleja nitida. Pharmaceutical Biology 46: 596–601.
Flamini, G. 2007. Flavonoids and other compounds from the aerial parts of Viola etrusca. Chemistry and Biodiversity 4: 139–144.
Giacobini, E. 2003. Cholinesterases: New roles in brain function and in Alzheimer’s disease. Neurochemical Research 28: 515–522.
Greig, N.H., T. Utsuki, D.K. Ingram, Y. Wang, G. Pepeu, C. Scali, Q.S. Yu, J. Mamczarz, H.W. Holloway, T. Giordano, D. Chen, K. Furukawa, K. Sambamurti, A. Brossi, and D.K. Lahiri. 2005. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proceedings of the National Academy of Sciences of the United States of America 102: 17213–17218.
Hasanein, P., and S. Shahidi. 2010. Effects of combined treatment with vitamins C and E on passive avoidance learning and memory in diabetic rats. Neurobiology of Learning and Memory 93: 472–478.
Ingkaninan, K., P. Temkitthawon, K. Chuenchom, T. Yuyaem, and W. Thongnoi. 2003. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. Journal of Ethnopharmacology 89: 261–264.
Ji, H.F., and H.Y. Zhang. 2006. Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. Journal of Molecular Structure 767: 3–9.
Kai, H., M. Baba, and T. Okuyama. 2007. Two new megastigmanes from the leaves of Cucumis sativus. Chemical and Pharmaceutical Bulletin 55: 133–136.
Kiem, P.V., C.V. Minh, H. Huong, J.J. Lee, I.S. Lee, and Y.H. Kim. 2005. Phenolic constituents with inhibitory activity against NFAT transcription from Desmos chinensis. Archives of Pharmacal Research 28: 1345–1349.
Khan, M., S.A.A. Elhussein, M.M. Khan, and N. Khan. 2012. Anti-acetylcholinesterase activity of Piper sarmentosum by a continuous immobilized-enzyme assay. APCBEE Procedia 2: 199–204.
Kou, X.J., and N. Chen. 2012. Pharmacological potential of ampelopsin in Rattan tea. Food Science and Human Wellness 1: 14–18.
Li, L., H. Sheng, F. Xu, and Q. Shen. 2009. Heterometal clusters Ln2Na8(OCH2CH2NMe2)12(OH)2 as homogeneous catalysts for the tishchenko reaction. Chinese Journal of Chemistry 27: 1127–1131.
Lim, S.S., S.M. Han, S.Y. Kim, Y.S. Bae, and I.J. Kang. 2007. Isolation of acetylcholinesterase inhibitors from the flowers of Chrysanthemum indicum Linne. Food Science and Biotechnology 16: 265–269.
Matsuda, H., T. Morikawa, J. Tao, K. Ueda, and M. Yoshikawa. 2002. Bioactive Constituents of Chinese Natural Medicines. VII. Inhibitors of degranulation in RBL-2H3 cells and absolute stereostructures of three new diarylheptanoid glycosides from the bark of Myrica rubra. Chemical & Pharmaceutical Bulletin 50: 208–215.
Michel, J., R.E. Duarte, J.L. Bolton, Y. Huang, A. Caceres, M. Veliz, D.D. Soejarto, and G.B. Mahady. 2007. Medical potential of plants used by the Q’eqchi Maya of Livingston, Guatemala for the treatment of women’s health complaints. Journal of Ethnopharmacology 114: 92–101.
Parihar, M.S., and T. Hemnani. 2004. Alzheimer’s disease pathogenesis and therapeutic interventions. Journal of Clinical Neuroscience 1: 456–467.
Peng, W.H., M.T. Hsieh, and C.R. Wu. 1997. Effect of long-term administration of berberine on scopolamine-induced amnesia in rats. Japanese Journal of Pharmacology 74: 261–266.
Philip, C.S., C.V. Nigel, and S.J.S. Monique. 2007. Polyoxygenated cyclohexane derivatives and other constituents from Kaempferia rotunda L. Phytochemistry 68: 1579–1586.
Scarpini, E., P. Scheltens, and H. Feldman. 2003. Treatment of Alzheimer’s disease: Current status and new perspectives. The Lancet Neurology 2: 539–547.
Sengupta, S., and A.B. Ray. 1987. The chemistry of Piper species: A review. Fitoterapia 58: 147–166.
Sawasdee, P., C. Sabphon, D. Sitthiwongwanit, and U. Kokpol. 2009. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytotherapy Research 23: 1792–1794.
Virinder, S.P., C.J. Subhash, S.B. Kirpal, J. Rajni, T. Poonam, J. Amitabh, D.T. Om, K.P. Ashok, W. Jesper, E.O. Carl, and B. Perm. 1997. Phytochemistry of the genus Piper. Phytochemistry 46: 597–673.
Whitehouse, P.J., D.L. Price, G.R. Struble, A.W. Clarke, J.T. Coyle, and M.R. DeLong. 1982. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 15: 1237–1239.
Zhu, F., and C. Qian. 2006. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1β and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC Neuroscience 7: 78–86.
Acknowledgments
This study was financially supported by the National Foundation for Science and Technology Development, Vietnam (Project No: 104.01-2010-16), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (BK21 Plus program; 22A20130000073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hoang Viet Dung and To Dao Cuong have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dung, H.V., Cuong, T.D., Chinh, N.M. et al. Compounds from the aerial parts of Piper bavinum and their anti-cholinesterase activity. Arch. Pharm. Res. 38, 677–682 (2015). https://doi.org/10.1007/s12272-014-0432-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0432-3