Abstract
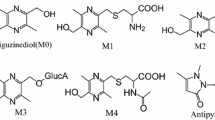
In this study, we determined the pharmacokinetics of mycophenolic acid (MPA) and its metabolites mycophenolic acid glucuronide (MPAG) and acyl glucuronide (AcMPAG) in rat plasma and bile, using a newly developed HPLC method. Protein precipitation and liquid-liquid extraction were employed in sample preparation of plasma and bile, respectively. The HPLC methods included a gradient elution consisting of acetonitrile and phosphate buffer at a flow rate of 1.2 mL/min, with UV detection at 254 nm. The HPLC method was found to be sensitive and linear (r 2 ≥ 0.9991, 1.0–128.0 and 0.25–32.0 mg/L for MPA; 1.0–128.0 and 0.5–64.0 mg/L for MPAG; 0.25–32.0 and 1.0–128.0 mg/L for AcMPAG in rat plasma and bile, respectively), precise (both the intra- and inter-day variability were ≤ 6.8%), and accurate (both the intra- and inter-day accuracy were between 92.2% and 105.4%). The average extraction efficiencies for MPA, MPAG and AcMPAG were 85.3%, 100.1%, and 94.7% in plasma, and 88.0%, 67.3%, and 68.3% in bile, respectively. The method was successfully employed for pharmacokinetic studies in plasma and bile after oral administration of mycophenolate mofetil (prodrug of MPA) in rats.
Similar content being viewed by others
References
Benoit-Biancamano, M. O., Caron, P., Lévesque, E., Delage, R., Couture, F., and Guillemette, C., Sensitive high-performance liquid chromatography-tandem mass spectrometry method for quantitative analysis of mycophenolic acid and its glucuronide metabolites in human plasma and urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 858, 159–167 (2007).
Bowalgaha, K. and Miners, J. O., The glucuronidation of mycophenolic mycopheacid by human liver, kidney and jejunum microsomes. Br. J. Clin. Pharmacol., 52, 605–609 (2001).
Brandhorst, G., Streit, F., Goetze, S., Oellerich, M., and Armstrong, V. W., Quantification by liquid chromatography tandem mass spectrometry of mycophenolic acid and its phenol and acyl glucuronide metabolites. Clin. Chem., 52, 1962–1964 (2006).
Bullingham, R. E., Nicholls, A., and Hale, M., Pharmacokinetics of mycophenolate mofetil (RS61443): a short review. Transplant. Proc., 28, 925–929 (1996).
Bunnapradist, S., Lentine, K. L., Burroughs, T. E., Pinsky, B. W., Hardinger, K. L., Brennan, D. C., and Schnitzler, M. A., Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation, 82, 102–107 (2006).
De Loor, H., Naesens, M., Verbeke, K., Vanrenterghem, Y., and Kuypers, D. R., Stability of mycophenolic acid and glucuronide metabolites in human plasma and the impact of deproteinization methodology. Clin. Chim. Acta, 389, 87–92 (2008).
Elbarbry, F. A. and Shoker, A. S., Liquid chromatographic determination of mycophenolic acid and its metabolites in human kidney transplant plasma: pharmacokinetic application. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 859, 276–281 (2007).
Jeong, H. and Kaplan, B., Therapeutic monitoring of mycophenolate mofetil. Clin. J. Am. Soc. Nephrol., 2, 184–191 (2007).
Khoschsorur, G. and Erwa, W., Liquid chromatographic method for simultaneous determination of mycophenolic acid and its phenol- and acylglucuronide metabolites in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 799, 355–360 (2004).
Knoll, G. A., MacDonald, I., Khan, A., and Van Walraven, C., Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J. Am. Soc. Nephrol., 14, 2381–2386 (2003).
Kuypers, D. R., Vanrenterghem, Y., Squifflet, J. P., Mourad, M., Abramowicz, D., Oellerich, M., Armstrong, V., Shipkova, M., and Daems, J., Twelve-month evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its glucuronide metabolites in renal allograft recipients on low dose Tacrolimus in combination with mycophenolate mofetil. Ther. Drug Monit., 25, 609–622 (2003).
Moore, J., Middleton, L., Cockwell, P., Adu, D., Ball, S., Little, M. A., Ready, A., Wheatley, K., and Borrows, R., Calcineurin inhibitor sparing with mycophenolate in kidney transplantation: a systematic review and metaanalysis. Transplantation, 87, 591–605 (2009).
Nowak, I. and Shaw, L. M., Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin. Chem., 41, 1011–1017 (1995).
Ohyama, K., Kishikawa, N., Nakagawa, H., Kuroda, N., Nishikido, M., Teshima, M., To, H., Kitahara, T., and Sasaki, H., Simultaneous determination of mycophenolic acid and its acyl and phenol glucuronide metabolites in human serum by capillary zone electrophoresis. J. Pharm. Biomed. Anal., 47, 201–206 (2008).
Patel, C. G., Mendonza, A. E., Akhlaghi, F., Majid, O., Trull, A. K., Lee, T., and Holt, D. W., Determination of total mycophenolic acid and its glucuronide metabolite using liquid chromatography with ultraviolet detection and unbound mycophenolic acid using tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 813, 287–294 (2004).
Patel, C. G. and Akhlaghi, F., High-performance liquid chromatographymethod for the determination of mycophenolic acid and its acyl and phenol glucuronide metabolites in human plasma. Ther. Drug Monit., 28, 116–122 (2006).
Patel, C. G., Harmon, M., Gohh, R. Y., and Akhlaghi, F., Concentrations of mycophenolic acid and glucuronide metabolites under concomitant therapy with cyclosporine or tacrolimus. Ther. Drug Monit., 29, 87–95 (2007).
Pillans, P. I., Rigby, R. J., Kubler, P., Willis, C., Salm, P., Tett, S. E., and Taylor, P. J., A retrospective analysis of mycophenolic acid and cyclosporin concentrations with acute rejection in renal transplant recipients. Clin. Biochem., 34, 77–81 (2001).
Saitoh, H., Kobayashi, M., Oda, M., Nakasato, K., Kobayashi, M., and Tadano, K., Characterization of intestinal absorption and enterohepatic circulation of mycophenolic Acid and its 7-O-glucuronide in rats. Drug Metab. Pharmacokinet., 21, 406–413 (2006).
Schütz, E., Shipkova, M., Armstrong, V. W., Wieland, E., and Oellerich, M., Identification of a pharmacologically active metabolite of mycophenolic acid in plasma of transplant recipients treated with mycophenolate mofetil. Clin. Chem., 45, 419–422 (1999).
Shipkova, M., Schütz, E., Armstrong, V. W., Niedmann, P. D., Wieland, E., and Oellerich, M., Overestimation of mycophenolic acid by EMIT correlates with MPA metabolite. Transplant. Proc., 31, 1135–1137 (1999).
Shipkova, M., Schütz, E., Armstrong, V. W., Niedmann, P. D., Oellerich, M., and Wieland, E., Determination of the acyl glucuronide metabolite of mycophenolic acid in human plasma by HPLC and Emit. Clin. Chem., 46, 365–372 (2000).
Staatz, C. E. and Tett, S. E., Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin. Pharmacokinet., 46, 13–58 (2007).
Sugioka, N., Koyama, H., Ohta, T., Kishimoto, H., Yasumura, T., and Takada, K., Pharmacokinetics of mycophenolate mofetil, a new immunosuppressant, in rats. J. Pharm. Sci., 85, 335–338 (1996).
Treinen-Moslen, M. and Kanz, M. F., Intestinal tract injury by drugs: Importance of metabolite delivery by yellow bile road. Pharmacol. Ther., 112, 649–667 (2006).
Westley, I. S., Brogan, L. R., Morris, R. G., Evans, A. M., and Sallustio, B. C., Role of Mrp2 in the hepatic disposition of mycophenolic acid and its glucuronide metabolites: effect of cyclosporine. Drug Metab. Dispos., 34, 261–266 (2006).
Yeung, S., Tong, K. L., Tsang, W. K., Tang, H. L., Fung, K. S., Chan, H. W., Chan, A. Y., and Chan, L., Determination of mycophenolate area under the curve by limited sampling strategy. Transplant. Proc., 33, 1052–1053 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Jw., Peng, Zh., Li, Xy. et al. simultaneous determination of mycophenolic acid and its metabolites by HPLC and pharmacokinetic studies in rat plasma and bile. Arch. Pharm. Res. 34, 59–69 (2011). https://doi.org/10.1007/s12272-011-0107-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-0107-2