Abstract
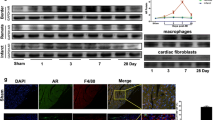
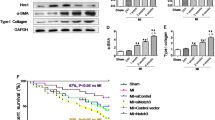
Transforming growth factor-β1 signaling pathways are known to involve in the development of post-infarction fibrosis, a process characterized by the aberrant activation, proliferation, and differentiation of fibroblasts, as well as the unbalanced turnover of extracellular matrix proteins. Recent studies have shown that Lefty1, a novel member of TGF-β superfamily, acts as a brake on the TGF-β signaling pathway in non-cardiac tissues. However, its role in myocardial infarction (MI)–induced fibrosis and left ventricular remodeling has not been fully elucidated. Here, for the first time, we reported that Lefty1 alleviated post-MI fibroblast proliferation, differentiation, and secretion through suppressing p-Smad2 and p-ERK1/2 signaling pathways in vivo and in vitro. In MI mice or TGF-β1-treated neonatal rat cardiac fibroblasts (CFBs), the expression of Lefty1 was upregulated. Adenovirus-mediated overexpression of Lefty1 significantly attenuated TGF-β1-induced CFBs’ proliferation, differentiation, and collagen production. Using the adeno-associated virus approach, we confirmed that Lefty1 attenuates MI-induced cardiac injury, as evidenced by the decreased infarct size and preserved cardiac function. These results highlight the importance of Lefty1 in the prevention of post-MI fibrosis and may help identify potential targets for therapeutic intervention of cardiac fibrosis.

Graphical abstract




Similar content being viewed by others
Abbreviations
- TGF-β1:
-
Transforming growth factor-β1
- MI:
-
Myocardial infarction
- CFB:
-
Cardiac fibroblast
- CF:
-
Cardiac fibrosis
- ECM:
-
Extracellular matrix
- HF:
-
Heart failure
- ALK:
-
Activin receptor–like kinase
- Smad:
-
Small mother against decapentaplegic
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphoinositide 3-kinase
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
c-Jun N-terminal kinase
- EMT:
-
Epithelial-mesenchymal transition
- UUO:
-
Unilateral ureteral obstruction
- rAAV9:
-
Recombinant adeno-associated virus serotype 9
- GFP:
-
Green fluorescent protein
- PBS:
-
Phosphate buffered saline
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- α-SMA:
-
α-smooth muscle actin
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD:
-
Optical density
- LVSD:
-
LV end-systolic diameter
- LVDD:
-
End-diastolic diameter
- EF:
-
Ejection fraction
- FS:
-
Fractional shortening
- BMP:
-
Bone morphogenetic protein
- GDF:
-
Growth differentiation factor
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
References
Talman, V., & Ruskoaho, H. (2016). Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell and Tissue Research, 365, 563–581.
Dobaczewski, M., Chen, W., & Frangogiannis, N. G. (2011). Transforming growth factor (TGF)-β signaling in cardiac remodeling. Journal of Molecular and Cellular Cardiology, 51, 600–606.
Heldin, C. H., Miyazono, K., & ten Dijke, P. (1997). TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature, 390, 465–471.
Euler-Taimor, G., & Heger, J. (2006). The complex pattern of SMAD signaling in the cardiovascular system. Cardiovascular Research, 69, 15–25.
Liu, G., Ma, C., Yang, H., & Zhang, P. Y. (2017). Transforming growth factor β and its role in heart disease. Experimental and Therapeutic Medicine, 13, 2123–2128.
Zhang, Y. E. (2017). Non-Smad signaling pathways of the TGF-β family. Cold Spring Harbor Perspectives in Biology, 9, a022129.
Kosaki, K., Bassi, M. T., Kosaki, R., Lewin, M., Belmont, J., Schauer, G., & Casey, B. (1999). Characterization and mutation analysis of human LEFTY A and LEFTY B, homologues of murine genes implicated in left-right axis development. American Journal of Human Genetics, 64, 712–721.
Mason, J. M., Xu, H. P., Rao, S. K., Leask, A., Barcia, M., Shan, J., Stephenson, R., & Tabibzadeh, S. (2002). Lefty contributes to the remodeling of extracellular matrix by inhibition of connective tissue growth factor and collagen mRNA expression and increased proteolytic activity in a fibrosarcoma model. The Journal of Biological Chemistry, 277, 407–415.
Xu, C., Xu, M., Wang, W., & Zhang, J. (2016). Lefty1 alleviates renal tubulointerstitial injury in mice with unilateral ureteral obstruction. Molecular Medicine Reports, 13, 901–908.
Zhang, L., Liu, X., Liang, J., Wu, J., Tan, D., & Hu, W. (2020). Lefty-1 inhibits renal epithelial-mesenchymal transition by antagonizing the TGF-β/Smad signaling pathway. Journal of Molecular Histology, 51, 77–87.
Fei, W., Kijima, D., Hashimoto, M., Hashimura, M., Oguri, Y., Kajita, S., Matsumoto, T., Yokoi, A., & Saegusa, M. (2017). A functional role of LEFTY during progesterone therapy for endometrial carcinoma. Cell Communication and Signaling: CCS, 15, 56.
Chen, Y. H., Wang, Q., Li, C. Y., Hou, J. W., Chen, X. M., Zhou, Q., Chen, J., Wang, Y. P., & Li, Y. G. (2017). Haplodeficiency of activin receptor-like kinase 4 alleviates myocardial infarction-induced cardiac fibrosis and preserves cardiac function. Journal of Molecular and Cellular Cardiology, 105, 1–11.
Ni, L., Scott Jr., L., Campbell, H. M., Pan, X., Alsina, K. M., Reynolds, J., Philippen, L. E., Hulsurkar, M., Lagor, W. R., Li, N., & Wehrens, X. H. T. (2019). Atrial-specific gene delivery using an adeno-associated viral vector. Circulation Research, 124, 256–262.
Morikawa, M., Derynck, R., & Miyazono, K. (2016). TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harbor Perspectives in Biology, 8, a021873.
Hanna, A., & Frangogiannis, N. G. (2019). The role of the TGF-β superfamily in myocardial infarction. Frontiers in Cardiovascular Medicine, 6, 140.
Hu, J., Wang, X., Wei, S. M., Tang, Y. H., Zhou, Q., & Huang, C. X. (2016). Activin A stimulates the proliferation and differentiation of cardiac fibroblasts via the ERK1/2 and p38-MAPK pathways. European Journal of Pharmacology, 789, 319–327.
Lin, J. F., Hsu, S. Y., Teng, M. S., Wu, S., Hsieh, C. A., Jang, S. J., Liu, C. J., Huang, H. L., & Ko, Y. L. (2016). Activin a predicts left ventricular remodeling and mortality in patients with ST-elevation myocardial infarction. Acta Cardiologica Sinica, 32, 420–427.
Sun, B., Huo, R., Sheng, Y., Li, Y., Xie, X., Chen, C., Liu, H. B., Li, N., Li, C. B., Guo, W. T., Zhu, J. X., Yang, B. F., & Dong, D. L. (2013). Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension, 61, 352–360.
Pachori, A. S., Custer, L., Hansen, D., Clapp, S., Kemppa, E., & Klingensmith, J. (2010). Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. Journal of Molecular and Cellular Cardiology, 48, 1255–1265.
Merino, D., Villar, A. V., García, R., Tramullas, M., Ruiz, L., Ribas, C., Cabezudo, S., Nistal, J. F., & Hurlé, M. A. (2016). BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovascular Research, 110, 331–345.
Zeisberg, E. M., Tarnavski, O., Zeisberg, M., Dorfman, A. L., McMullen, J. R., Gustafsson, E., Chandraker, A., Yuan, X., Pu, W. T., Roberts, A. B., Neilson, E. G., Sayegh, M. H., Izumo, S., & Kalluri, R. (2007). Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature Medicine, 13, 952–961.
Ren, Q., Lin, P., Wang, Q., Zhang, B., & Feng, L. (2019). Chronic peripheral ghrelin injection exerts antifibrotic by increasing growth differentiation factor 15 in rat hearts with myocardial fibrosis induced by isoproterenol. Physiological Research, 69, 439–450.
Meno, C., Saijoh, Y., Fujii, H., Ikeda, M., Yokoyama, T., Yokoyama, M., Toyoda, Y., & Hamada, H. (1996). Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature, 381, 151–155.
Zhang, L., Zhang, J., Xu, C., Zhou, X., Wang, W., Zheng, R., Hu, W., & Wu, P. (2015). Lefty-1 alleviates TGF-β1-induced fibroblast-myofibroblast transdifferentiation in NRK-49F cells. Drug Design, Development and Therapy, 9, 4669–4678.
Ma, H., Qiao, S., Wang, Z., Geng, S., Zhao, Y., Hou, X., Tian, W., Chen, X., & Yao, L. (2017). Microencapsulation of Lefty-secreting engineered cells for pulmonary fibrosis therapy in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology, 312, L741–l747.
Ulloa, L., & Tabibzadeh, S. (2001). Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. The Journal of Biological Chemistry, 276, 21397–21404.
Gao, L., Wang, L. Y., Liu, Z. Q., Jiang, D., Wu, S. Y., Guo, Y. Q., Tao, H. M., Sun, M., You, L. N., Qin, S., Cheng, X. C., Xie, J. S., Chang, G. L., & Zhang, D. Y. (2020). TNAP inhibition attenuates cardiac fibrosis induced by myocardial infarction through deactivating TGF-β1/Smads and activating P53 signaling pathways. Cell Death & Disease, 11, 44.
Chen, L. L., Yin, H., & Huang, J. (2007). Inhibition of TGF-beta1 signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through angiogenesis and reduction of apoptosis. Cardiovascular Pathology, 16, 221–230.
Huang, W., Rubinstein, J., Prieto, A. R., & Wang, D. H. (2010). Enhanced postmyocardial infarction fibrosis via stimulation of the transforming growth factor-beta-Smad2 signaling pathway: role of transient receptor potential vanilloid type 1 channels. Journal of Hypertension, 28, 367–376.
Liu, H., Liu, A., Shi, C., & Li, B. (2016). Curcumin suppresses transforming growth factor-β1-induced cardiac fibroblast differentiation via inhibition of Smad-2 and p38 MAPK signaling pathways. Experimental and Therapeutic Medicine, 11, 998–1004.
Wu, H., Li, G. N., Xie, J., Li, R., Chen, Q. H., Chen, J. Z., Wei, Z. H., Kang, L. N., & Xu, B. (2016). Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovascular Disorders, 16, 5.
Wang, Y., Guo, Z., Gao, Y., Liang, P., Shan, Y., & He, J. (2019). Angiotensin II receptor blocker LCZ696 attenuates cardiac remodeling through the inhibition of the ERK signaling pathway in mice with pregnancy-associated cardiomyopathy. Cell & Bioscience, 9, 86.
Luo, S., Hieu, T. B., Ma, F., Yu, Y., Cao, Z., Wang, M., Wu, W., Mao, Y., Rose, P., Law, B. Y., & Zhu, Y. Z. (2017). ZYZ-168 alleviates cardiac fibrosis after myocardial infarction through inhibition of ERK1/2-dependent ROCK1 activation. Scientific Reports, 7, 43242.
Pan, Z., Zhao, W., Zhang, X., Wang, B., Wang, J., Sun, X., Liu, X., Feng, S., Yang, B., & Lu, Y. (2011). Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. British Journal of Pharmacology, 162, 688–700.
Funding
This study was supported by Grants from the National Natural Science Foundation of China (No. 81700271) and S&T Program of Hebei (No. H2018105054).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All experimental procedures were conducted in compliance with both the Animal Care and Use Committee of Capital Medical University and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (the 8th Edition, NRC 2011). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Cy., Zhang, Jr., Li, Xx. et al. Lefty1 Ameliorates Post-infarction Fibrosis by Suppressing p-Smad2 and p-ERK1/2 Signaling Pathways. J. of Cardiovasc. Trans. Res. 14, 636–646 (2021). https://doi.org/10.1007/s12265-020-10089-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10089-2