Abstract
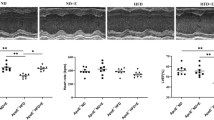
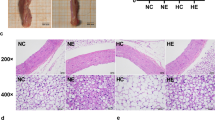
Previously, we found that miR-492 delayed the progression of atherosclerosis (AS) by acting as an up-stream regulator of resistin. Therefore, we hypothesized that the anti-atherogenic effects of exercise are related to miR-492-mediated downregulation of resistin and repair of endothelial injury. In this study, we investigated the effects of the miR-492/resistin axis on improving endothelial injury in ApoE−/− mice (ApoE-deficient/knockout in C57BL/6 mice) through swimming exercises. Our results showed that the severity of AS and insulin resistance (IR) in these mice were significantly reduced by swimming exercises. In addition, miR-492 expression in the aortic endothelium of ApoE−/− mice was decreased, in addition to increased levels of resistin. Interestingly, swimming exercises increased miR-492 expression while decreasing that of resistin. Taken together, swimming exercises delayed the progression of AS, possibly by upregulating miR-492 and downregulating resistin in aortic endothelium. Therefore, exercises modulated glucose and lipid metabolism, alleviated endothelial IR, and repaired endothelial injury.



Similar content being viewed by others
References
Tian, F., Yu, B. L., & Hu, J. R. (2014). mTOR mediates the cross-talk of macrophage polarization and autophagy in atherosclerosis. International Journal of Cardiology, 177(1), 144–145. https://doi.org/10.1016/j.ijcard.2014.09.035.
Sun, C. H., Cai, Y., Zhang, W. L., Xie, K. L., Liu, Y., & Liu, S. X. (2013). The effects of swimming training on expression of PPAR-γ,CPT-1 and MCAD in ApoE knock-out mice. Chinese Journal of Rehabilitation Medicine, 28(2), 134–138.
Li, J. (2011). The effects of swimming training on myocardial PPAR-γ expression and glucose and lipid metabolism in mice with insulin resistance [D]. Changsha: Central South University.
Liu, H., Jiang, D., Zhang, S., & Ou, B. (2010). Aspirin inhibits fractalkine expression in atherosclerotic plaques and reduces atherosclerosis in ApoE gene knockout mice. Cardiovascular Drugs and Therapy, 24(1), 17–24. https://doi.org/10.1007/s10557-009-6210-7.
Muniyappa, R., Montagnani, M., Koh, K. K., & Quon, M. J. (2007). Cardiovascular actions of insulin. Endocrine Reviews, 28(5), 463–491. https://doi.org/10.1210/er.2007-0006.
Muniyappa, R., & Sowers, J. R. (2013). Role of insulin resistance in endothelial dysfunction. Reviews in Endocrine & Metabolic Disorders, 14(1), 5–12. https://doi.org/10.1007/s11154-012-9229-1.
Zhang, Y., Li, L., You, J., Cao, J., & Fu, X. (2013). Effect of 7-difluoromethyl-5, 4′-dimethoxygenistein on aorta atherosclerosis in hyperlipidemia ApoE(-/-) mice induced by a cholesterol-rich diet. Drug Design, Development and Therapy, 7, 233–242. https://doi.org/10.2147/DDDT.S37512.
Melo, S. F., Fernandes, T., Barauna, V. G., Matos, K. C., Santos, A. A., Tucci, P. J., et al. (2014). Expression of MicroRNA-29 and collagen in cardiac muscle after swimming training in myocardial-infarcted rats. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 33(3), 657–669. https://doi.org/10.1159/000358642.
Wu, X. D., Zeng, K., Liu, W. L., Gao, Y. G., Gong, C. S., Zhang, C. X., et al. (2014). Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. International Journal of Sports Medicine, 35(4), 344–350. https://doi.org/10.1055/s-0033-1349075.
Cai, Y., Liu, S. X., Xie, K. L., Zhang, W. L., Dong, L., Liu, Y., et al. (2014). MicroRNA-492 reverses high glucose-induced insulin resistance in HUVEC cells through targeting resistin. Molecular and Cellular Biochemistry, 391(1–2), 117–125. https://doi.org/10.1007/s11010-014-1993-7.
Li, Q., Su, J., Jin, S. J., Wei, W., Cong, X. D., Li, X. X., et al. (2018). Argirein alleviates vascular endothelial insulin resistance through suppressing the activation of Nox4-dependent O2(-) production in diabetic rats. Free Radical Biology & Medicine, 121, 169–179. https://doi.org/10.1016/j.freeradbiomed.2018.04.573.
Nakashima, Y., Raines, E. W., Plump, A. S., Breslow, J. L., & Ross, R. (1998). Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arteriosclerosis, Thrombosis, and Vascular Biology, 18(5), 842–851.
Nakashima, Y., Plump, A. S., Raines, E. W., Breslow, J. L., & Ross, R. (1994). ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis and Thrombosis : A Journal of Vascular Biology, 14(1), 133–140.
Cheng, J., Song, J., He, X., Zhang, M., Hu, S., Zhang, S., et al. (2017). Erratum. Loss of Mbd2 protects mice against high-fat diet-induced obesity and insulin resistance by regulating the homeostasis of energy storage and expenditure. Diabetes 2016;65:3384-3395. Diabetes, 66(9), 2531. https://doi.org/10.2337/db17-er09b.
Huang, X. S., Zhao, S. P., Hu, M., Bai, L., Zhang, Q., & Zhao, W. (2010). Decreased apolipoprotein A5 is implicated in insulin resistance-related hypertriglyceridemia in obesity. Atherosclerosis, 210(2), 563–568. https://doi.org/10.1016/j.atherosclerosis.2009.12.004.
Zhang, S. H., Reddick, R. L., Piedrahita, J. A., & Maeda, N. (1992). Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science, 258(5081), 468–471.
Chen, Y., Yang, G. Y., & Ling, L. I. (2009). The changes of glucose-lipid metabolism in apoe~(-/-) mice with high-fat induced insulin resistance. Chinese Journal of Diabetes, 17(8), 590–593.
Su, Z., Li, Y., James, J. C., Matsumoto, A. H., Helm, G. A., Lusis, A. J., et al. (2006). Genetic linkage of hyperglycemia, body weight and serum amyloid-P in an intercross between C57BL/6 and C3H apolipoprotein E-deficient mice. Human Molecular Genetics, 15(10), 1650–1658. https://doi.org/10.1093/hmg/ddl088.
Ruderman, N. B., & Haudenschild, C. (1984). Diabetes as an atherogenic factor. Progress in Cardiovascular Diseases, 26(5), 373–412.
von Eckardstein, A., Schulte, H., & Assmann, G. (2000). Risk for diabetes mellitus in middle-aged Caucasian male participants of the PROCAM study: Implications for the definition of impaired fasting glucose by the American Diabetes Association. Prospective Cardiovascular Munster. The Journal of Clinical Endocrinology and Metabolism, 85(9), 3101–3108. https://doi.org/10.1210/jcem.85.9.6773.
Kendall, D. M. (2005). The dyslipidemia of diabetes mellitus: Giving triglycerides and high-density lipoprotein cholesterol a higher priority? Endocrinology and Metabolism Clinics of North America, 34(1), 27–48. https://doi.org/10.1016/j.ecl.2004.11.004.
Tian, J., Pei, H., James, J. C., Li, Y., Matsumoto, A. H., Helm, G. A., et al. (2005). Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility. Biochemical and Biophysical Research Communications, 329(3), 1102–1107. https://doi.org/10.1016/j.bbrc.2005.02.090.
Liu, Y., Wang, Q., Pan, Y. B., Gao, Z. J., Liu, Y. F., & Chen, S. H. (2008). Effects of over-expressing resistin on glucose and lipid metabolism in mice. Journal of Zhejiang University. Science. B, 9(1), 44–50. https://doi.org/10.1631/jzus.B071479.
Moon, B., Kwan, J. J., Duddy, N., Sweeney, G., & Begum, N. (2003). Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. American Journal of Physiology. Endocrinology and Metabolism, 285(1), E106–E115. https://doi.org/10.1152/ajpendo.00457.2002.
Palanivel, R., & Sweeney, G. (2005). Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Letters, 579(22), 5049–5054. https://doi.org/10.1016/j.febslet.2005.08.011.
Sato, N., Kobayashi, K., Inoguchi, T., Sonoda, N., Imamura, M., Sekiguchi, N., et al. (2005). Adenovirus-mediated high expression of resistin causes dyslipidemia in mice. Endocrinology, 146(1), 273–279. https://doi.org/10.1210/en.2004-0985.
Kushiyama, A., Sakoda, H., Oue, N., Okubo, M., Nakatsu, Y., Ono, H., et al. (2013). Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(8), 1986–1993. https://doi.org/10.1161/ATVBAHA.113.301546.
Wang, J. M., Tao, J., Chen, D. D., Cai, J. J., Irani, K., Wang, Q., et al. (2014). MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(1), 99–109. https://doi.org/10.1161/ATVBAHA.113.302104.
Poliseno, L., Tuccoli, A., Mariani, L., Evangelista, M., Citti, L., Woods, K., et al. (2006). MicroRNAs modulate the angiogenic properties of HUVECs. Blood, 108(9), 3068–3071. https://doi.org/10.1182/blood-2006-01-012369.
Minami, Y., Satoh, M., Maesawa, C., Takahashi, Y., Tabuchi, T., Itoh, T., et al. (2009). Effect of atorvastatin on microRNA 221/222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. European Journal of Clinical Investigation, 39(5), 359–367. https://doi.org/10.1111/j.1365-2362.2009.02110.x.
Liu, X., Cheng, Y., Zhang, S., Lin, Y., Yang, J., & Zhang, C. (2009). A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circulation Research, 104(4), 476–487. https://doi.org/10.1161/CIRCRESAHA.108.185363.
Chen, T., Huang, Z., Wang, L., Wang, Y., Wu, F., Meng, S., et al. (2009). MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovascular Research, 83(1), 131–139. https://doi.org/10.1093/cvr/cvp121.
Zhang, Q., Liu, L., & Zheng, X. Y. (2009). Protective roles of HDL, apoA-I and mimetic peptide on endothelial function: Through endothelial cells and endothelial progenitor cells. International Journal of Cardiology, 133(3), 286–292. https://doi.org/10.1016/j.ijcard.2008.11.034.
Patella, F., Leucci, E., Evangelista, M., Parker, B., Wen, J., Mercatanti, A., et al. (2013). MiR-492 impairs the angiogenic potential of endothelial cells. Journal of Cellular and Molecular Medicine, 17(8), 1006–1015. https://doi.org/10.1111/jcmm.12085.
Adams, V., Linke, A., Krankel, N., Erbs, S., Gielen, S., Mobius-Winkler, S., et al. (2005). Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation, 111(5), 555–562. https://doi.org/10.1161/01.CIR.0000154560.88933.7E.
de Beer, V. J., Bender, S. B., Taverne, Y. J., Gao, F., Duncker, D. J., Laughlin, M. H., et al. (2011). Exercise limits the production of endothelin in the coronary vasculature. American Journal of Physiology. Heart and Circulatory Physiology, 300(5), H1950–H1959. https://doi.org/10.1152/ajpheart.00954.2010.
Ekblom-Bak, E., Hellenius, M. L., Ekblom, O., Engstrom, L. M., & Ekblom, B. (2010). Independent associations of physical activity and cardiovascular fitness with cardiovascular risk in adults. European Journal of Cardiovascular Prevention and Rehabilitation : Official Journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology, 17(2), 175–180. https://doi.org/10.1097/HJR.0b013e32833254f2.
Andersen, L. B., Riddoch, C., Kriemler, S., & Hills, A. P. (2011). Physical activity and cardiovascular risk factors in children. British Journal of Sports Medicine, 45(11), 871–876. https://doi.org/10.1136/bjsports-2011-090333.
Gielen, S., Schuler, G., & Hambrecht, R. (2001). Exercise training in coronary artery disease and coronary vasomotion. Circulation, 103(1), E1–E6.
Perk, J., De Backer, G., Gohlke, H., Graham, I., Reiner, Z., Verschuren, M., et al. (2012). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European Heart Journal, 33(13), 1635–1701. https://doi.org/10.1093/eurheartj/ehs092.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Funding
This work was supported by the Natural Science Foundation of Hunan Province (Grant number: 2016JJ6160) and the National Natural Science Foundation of China (Grant number: 81501955).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All animal experiments in this study complied with the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Ethics Committee of Central South University. All efforts were made to minimize the suffering of the animals included in the current study.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Rights and permissions
About this article
Cite this article
Cai, Y., Xie, KL., Zheng, F. et al. Aerobic Exercise Prevents Insulin Resistance Through the Regulation of miR-492/Resistin Axis in Aortic Endothelium. J. of Cardiovasc. Trans. Res. 11, 450–458 (2018). https://doi.org/10.1007/s12265-018-9828-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-018-9828-7