Abstract
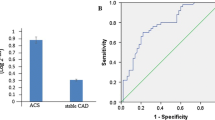
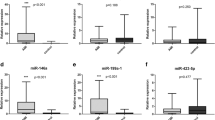
Recent studies have suggested that serum microRNAs (miRNAs) are novel biomarkers for many cardiovascular diseases, but their role in Kawasaki disease (KD) is still unclear. We demonstrated that serum miR-92a-3p levels were significantly higher in children with KD compared with children with fever and controls (both P < 0.05). When the disease recovered, miR-92a-3p levels returned to those of controls. Clinical and pathological data showed that high levels of miR-92a-3p were significantly associated with coronary artery lesions (CALs). Analysis of the receiver operating characteristic (ROC) curve showed that serum miR-92a-3p had a sensitivity of 81.8% and a specificity of 66.7% for distinguishing KD with CALs from KD without CALs. The area under the curve was 0.816 (P < 0.05, 95% CI 0.669–0.962). Therefore, the miRNA miR-92a-3p may be used as a potential biomarker for diagnosis of KD and KD with coronary artery lesions.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CALs:
-
Coronary artery lesions
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- FGF:
-
Fibroblast growth factor
- HUVECs:
-
Human umbilical vein endothelial cells
- KD:
-
Kawasaki disease
- miRNAs:
-
MicroRNAs
- NT-proBNP:
-
N-terminal pro natriuretic peptide type B
- RT-PCR:
-
Real-time reverse transcription-PCR
- WBC:
-
White blood cell
- ROC:
-
Receiver operating characteristic
References
Kawasaki, T., Kosaki, F., Okawa, S., Shigematsu, I., & Yanagawa, H. (1974). A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics, 54, 271–276.
Cheung, Y. F. (2014). Vascular health late after Kawasaki disease: implications for accelerated atherosclerosis. Korean Journal of Pediatrics, 57, 472–478.
Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116, 281–297.
Lagos-Quintana, M., Rauhut, R., Lendeckel, W., & Tuschl, T. (2001). Identification of novel genes coding for small expressed RNAs. Science, 294, 853–858.
Calin, G. A., Sevignani, C., Dumitru, C. D., Hyslop, T., Noch, E., Yendamuri, S., Shimizu, M., Rattan, S., Bullrich, F., Negrini, M., & Croce, C. M. (2004). Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proceedings of the National Academy of Sciences of the United States of America, 101, 2999–3004.
Lewis, B. P., Burge, C. B., & Bartel, D. P. (2005). Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell, 120, 15–20.
Yun, K. W., Lee, J. Y., Yun, S. W., Lim, I. S., & Choi, E. S. (2014). Elevated serum level of microRNA (miRNA)-200c and miRNA-371-5p in children with Kawasaki disease. Pediatric Cardiology, 35, 745–752.
Rowley, A. H., Pink, A. J., Reindel, R., Innocentini, N., Baker, S. C., Shulman, S. T., & Kim, K. Y. (2014). A study of cardiovascular miRNA biomarkers for Kawasaki disease. Pediatric Infectious Disease Journal, 33, 1296–1299.
Shimizu, C., Kim, J., Stepanowsky, P., Trinh, C., Lau, H. D., Akers, J. C., Chen, C., Kanegaye, J. T., Tremoulet, A., Ohno-Machado, L., & Burns, J. C. (2013). Differential expression of miR-145 in children with Kawasaki disease. PLoS One, 8, e58159.
Ni, F. F., Li, C. R., Li, Q., Xia, Y., Wang, G. B., & Yang, J. (2014). Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clinical and Experimental Immunology, 178, 384–393.
Newburger, J. W., Takahashi, M., Gerber, M. A., Gewitz, M. H., Tani, L. Y., Burns, J. C., Shulman, S. T., Bolger, A. F., Ferrieri, P., Baltimore, R. S., et al. (2004). Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics, 114, 1708–1733.
Liao, Y. C., Wang, Y. S., Guo, Y. C., Lin, W. L., Chang, M. H., & Juo, S. H. (2014). Let-7g improves multiple endothelial functions through targeting transforming growth factor-beta and SIRT-1 signaling. Journal of the American College of Cardiology, 63, 1685–1694.
Bao, M. H., Feng, X., Zhang, Y. W., Lou, X. Y., Cheng, Y., & Zhou, H. H. (2013). Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. International Journal of Molecular Sciences, 14, 23086–23102.
Tung, Y. T., Huang, P. W., Chou, Y. C., Lai, C. W., Wang, H. P., Ho, H. C., Yen, C. C., Tu, C. Y., Tsai, T. C., Yeh, D. C., et al. (2015). Lung tumorigenesis induced by human vascular endothelial growth factor (hVEGF)-A165 overexpression in transgenic mice and amelioration of tumor formation by miR-16. Oncotarget, 6, 10222–10238.
Song, D. W., Ryu, J. Y., Kim, J. O., Kwon, E. J., & Kim do, H. (2014). The miR-19a/b family positively regulates cardiomyocyte hypertrophy by targeting atrogin-1 and MuRF-1. The Biochemical Journal, 457, 151–162.
Xue, Y., Wei, Z., Ding, H., Wang, Q., Zhou, Z., Zheng, S., Zhang, Y., Hou, D., Liu, Y., Zen, K., et al. (2015). MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1alpha in the progression of atherosclerosis. Atherosclerosis, 241, 671–681.
Jia, C., Xiong, M., Wang, P., Cui, J., Du, X., Yang, Q., Wang, W., Chen, Y., & Zhang, T. (2014). Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS One, 9, e99849.
Pin, A. L., Houle, F., Guillonneau, M., Paquet, E. R., Simard, M. J., & Huot, J. (2012). miR-20a represses endothelial cell migration by targeting MKK3 and inhibiting p38 MAP kinase activation in response to VEGF. Angiogenesis, 15, 593–608.
Chen, C. F., Huang, J., Li, H., Zhang, C., Huang, X., Tong, G., & Xu, Y. Z. (2015). MicroRNA-221 regulates endothelial nitric oxide production and inflammatory response by targeting adiponectin receptor 1. Gene, 565, 246–251.
Yu S, Hong Q, Wang Y, Hou K, Wang L, Zhang Y, Fu B, Zhou Y, Zheng W, Chen X, Wu D: High concentrations of uric acid inhibit angiogenesis via regulation of the Krüppel-like factor 2-vascular endothelial growth factor-A axis by miR-92a. Circ J, 2015.
Wu, W., Xiao, H., Laguna-Fernandez, A., Villarreal, G., Jr., Wang, K. C., Geary, G. G., Zhang, Y., Wang, W. C., Huang, H. D., Zhou, J., et al. (2011). Flow-dependent regulation of Krüppel-like factor 2 is mediated by microRNA-92a. Circulation, 124, 633–641.
Zhao, L. L., Wang, Y. B., & Suo, L. (2011). Meta-analysis of the risk factors for coronary artery lesion secondary to Kawasaki disease in Chinese children. Zhonghua Er Ke Za Zhi, 49, 459–467.
Nakano, H., Ueda, K., Saito, A., Tsuchitani, Y., Kawamori, J., Miyake, T., & Yoshida, T. (1986). Scoring method for identifying patients with Kawasaki disease at high risk of coronary artery aneurysms. American Journal of Cardiology, 58, 739–742.
Kaneko, K., Yoshimura, K., Ohashi, A., Kimata, T., Shimo, T., & Tsuji, S. (2011). Prediction of the risk of coronary arterial lesions in Kawasaki disease by brain natriuretic peptide. Pediatric Cardiology, 32, 1106–1109.
Masi, L., Franceschelli, F., Leoncini, G., Gozzini, A., Rigante, D., La Torre, F., Matucci-Cerinic, M., Brandi, M. L., & Falcini, F. (2013). Can fibroblast growth factor (FGF)-23 circulating levels suggest coronary artery abnormalities in children with Kawasaki disease? Clinical and Experimental Rheumatology, 31, 149–153.
Hartopo, A. B., & Setianto, B. Y. (2013). Coronary artery sequel of Kawasaki disease in adulthood, a concern for internists and cardiologists. Acta Medica Indonesiana, 45, 69–75.
Honardoost, M. A., Kiani-Esfahani, A., Ghaedi, K., Etemadifar, M., & Salehi, M. (2014). miR-326 and miR-26a, two potential markers for diagnosis of relapse and remission phases in patient with relapsing-remitting multiple sclerosis. Gene, 544, 128–133.
Ries, J., Vairaktaris, E., Agaimy, A., Kintopp, R., Baran, C., Neukam, F. W., & Nkenke, E. (2014). miR-186, miR-3651 and miR-494: potential biomarkers for oral squamous cell carcinoma extracted from whole blood. Oncology Reports, 31, 1429–1436.
Aguado-Fraile, E., Ramos, E., Conde, E., Rodriguez, M., Martin-Gomez, L., Lietor, A., Candela, A., Ponte, B., Liano, F., & Garcia-Bermejo, M. L. (2015). A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PLoS One, 10, e0127175.
Tan, Y., Pan, T., Ye, Y., Ge, G., Chen, L., Wen, D., & Zou, S. (2014). Serum microRNAs as potential biomarkers of primary biliary cirrhosis. PLoS One, 9, e111424.
Ren, J., Zhang, J., Xu, N., Han, G., Geng, Q., Song, J., Li, S., Zhao, J., & Chen, H. (2013). Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One, 8, e80738.
Rippe, C., Blimline, M., Magerko, K. A., Lawson, B. R., LaRocca, T. J., Donato, A. J., & Seals, D. R. (2012). MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Experimental Gerontology, 47, 45–51.
Loyer, X., Potteaux, S., Vion, A. C., Guerin, C. L., Boulkroun, S., Rautou, P. E., Ramkhelawon, B., Esposito, B., Dalloz, M., Paul, J. L., et al. (2014). Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circulation Research, 114, 434–443.
Niculescu, L. S., Simionescu, N., Sanda, G. M., Carnuta, M. G., Stancu, C. S., Popescu, A. C., Popescu, M. R., Vlad, A., Dimulescu, D. R., Simionescu, M., & Sima, A. V. (2015). MiR-486 and miR-92a identified in circulating HDL discriminate between stable and vulnerable coronary artery disease patients. PLoS One, 10, e0140958.
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D., & Remaley, A. T. (2011). MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology, 13, 423–433.
Yim, D., Curtis, N., Cheung, M., & Burgner, D. (2013). Update on Kawasaki disease: epidemiology, aetiology and pathogenesis. Journal of Paediatrics and Child Health, 49, 704–708.
Acknowledgements
The technical assistance of Jingjing Zeng is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures followed were in accordance with the ethical standards of the Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical Ethics Committee. Informed consent was obtained from all patients for being included in the study.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY12H02003, LQ15H020006), the Zhejiang Provincial Medical and Health Science and Technology Plan (2012ZDA035), the Science and Technology Department of Zhejiang Province (2015C33163), and the Scientific Research Foundation of Wenzhou (Y20140051).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Additional information
Xing Rong and Lianhong Jia contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rong, X., Jia, L., Hong, L. et al. Serum miR-92a-3p as a New Potential Biomarker for Diagnosis of Kawasaki Disease with Coronary Artery Lesions. J. of Cardiovasc. Trans. Res. 10, 1–8 (2017). https://doi.org/10.1007/s12265-016-9717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-016-9717-x