Abstract
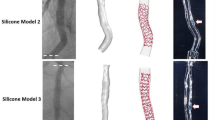
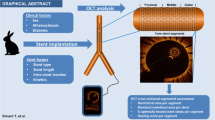
Current in vivo models of arterial lesions often lead to unpredictable results in terms of lesion anatomy and hemodynamical significance. This study aimed to evaluate the impact of coronary stenosis using a novel in vivo adjustable stenosis model capable of mimicking advanced human coronary lesions. We developed a series of balloon expandable covered coronary stents with a central restriction, mimicking different intermediate to severe stenosis, and implanted them percutaneously in coronary arteries of eight healthy hybrid Landrace pigs. Optical coherence tomography (OCT) pullbacks and fractional flow reserve (FFR) were acquired along the artery after implantation of the stenotic stents for precise evaluation of anatomy and functional impact. Diameter and area stenosis after deployment of the stenosis implant were, on average, respectively, 54.1 ± 5.9 and 78.4 ± 5.8 % and average FFR value was 0.83 (SD 0.13). There was a low correlation between FFR and MLA evaluated by OCT (r = 0.02, p = 0.94), improved with percentage area stenosis (r = −0.55, p = 0.12), or OCT volumetric evaluation of the stenosis taking into account not only the MLA but also the length of the lesion (r = −0.78, p = 0.01). This study presents a method and proof of concept for percutaneously introducing, and removing, anatomical stenosis of predetermined severity in vivo. Such in vivo model may be used to create and evaluate the impact of focal stenoses on physiological parameters such as FFR.






Similar content being viewed by others
Abbreviations
- CAD:
-
Coronary artery diseases
- CFD:
-
Computational flow dynamic
- CT:
-
Computed tomography
- IVUS:
-
Intravascular ultrasound
- FFR:
-
Fractional flow reserve
- MLD:
-
Minimum lumen diameter
- MLA:
-
Minimum lumen area
- MLV:
-
Minimum lumen volume
- OCT:
-
Optical coherence tomography
- QCA:
-
Quantitative coronary angiography
References
Suzuki, Y., Yeung, A. C., & Ikeno, F. (2009). The pre-clinical animal model in the translational research of interventional cardiology. JACC Cardiovascular Interventions, 2, 373–383.
FDA. General considerations for animal studies for cardiovascular devices. Guidance for Industry and FDA Staff 2010;Center for Devices and Radiological Health.
Pijls, N., van Son, J., Kirkeeide, R., De Bruyne, B., & Gould, K. (1993). Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation, 87, 1354–1367.
Folts, J., Crowell, E., Jr., & Rowe, G. (1976). Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation, 54, 365–370.
Schaper, W., Schaper, J., Xhonneux, R., & Vandesteene, R. (1969). The morphology of intercoronary anastomoses in chronic coronary artery occlusion. Cardiovascular Research, 3, 315–323.
Roth, D. M., Maruoka, Y., Rogers, J., White, F. C., Longhurst, J. C., & Bloor, C. M. (1987). Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. American Journal of Physiology. Heart and Circulatory Physiology, 253, H1279–H1288.
Hughes, G. C., Post, M. J., Simons, M., & Annex, B. H. (2003). Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. Journal of Applied Physiology, 94, 1689–1701.
Litvak, J., Siderides, L. E., & Vineberg, A. M. (1957). The experimental production of coronary artery insufficiency and occlusion. American Heart Journal, 53, 505–518.
von Georges, D., Philip, R., Christian, K., Corinna, L., Rabea, H., Franz Josef, G., Wolfgang, M. N., Andrea, K., Johannes, W., Marcus, S., Markus, S., Eckart, T., & Peter, B. (2003). Selective pressure-regulated retroinfusion of fibroblast growth factor-2 into the coronary vein enhances regional myocardial blood flow and function in pigs with chronic myocardial ischemia. Journal of the American College of Cardiology, 42, 1120–1128.
Lazoura O, Zacharoulis D, Kanavou T, Rountas C, Katsimboulas M, Tzovaras G, Habib N. A novel experimental animal model of arterial stenosis based on endovascular radiofrequency energy application. Journal of Investigative Surgery.24:123-128
Suzuki, Y. (2008). The porcine restenosis model using thermal balloon injury: comparison with the model by coronary stenting. The Journal of Invasive Cardiology, 20, 142.
Wu, M., Bogaert, J., D'hooge, J., Sipido, K., Maes, F., Dymarkowski, S., Rademakers, F., & Claus, P. (2010). Closed-chest animal model of chronic coronary artery stenosis. Assessment with magnetic resonance imaging. The International Journal of Cardiovascular Imaging, 26, 299–308. formerly Cardiac Imaging.
Staab, M. E., Meeker, D. K., Edwards, W. D., Camrud, A. R., Jorgenson, M. A., Camrud, L. J., Srivatsa, S. S., Jeong, M. H., Gregoire, J., Holmes, D. R., & Schwartz, R. S. (1997). Reliable models of severe coronary stenosis in porcine coronary arteries: lesion induction by high temperature or copper stent. Journal of Interventional Cardiology, 10, 61–69.
Mintz, G. S., Nissen, S. E., Anderson, W. D., Bailey, S. R., Erbel, R., Fitzgerald, P. J., Pinto, F. J., Rosenfield, K., Siegel, R. J., Tuzcu, E. M., Yock, P. G., O’Rourke, R. A., Abrams, J., Bates, E. R., Brodie, B. R., Douglas, P. S., Gregoratos, G., Hlatky, M. A., Hochman, J. S., Kaul, S., Tracy, C. M., Waters, D. D., & Winters, W. L., Jr. (2001). American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS): a report of the american college of cardiology task force on clinical expert consensus documents developed in collaboration with the european society of cardiology endorsed by the society of cardiac angiography and interventions. Journal of the American College of Cardiology, 37, 1478–1492.
Yong ASC, Ng ACC, Brieger D, Lowe HC, Ng MKC, Kritharides L. Three-dimensional and two-dimensional quantitative coronary angiography, and their prediction of reduced fractional flow reserve. European Heart Journal. 2010.
Kolozsvári R, Tar B, Lugosi P, Sánta J, Béres Z, Ungvári T, Polgár P, Kőszegi Z. Plaque volume derived from three-dimensional reconstruction of coronary angiography predicts the fractional flow reserve. International Journal of Cardiology. 2011;in press.
Francis DP. How easily can omission of patients, or selection amongst poorly-reproducible measurements, create artificial correlations? Methods for detection and implications for observational research design in cardiology. International Journal of Cardiology.
Sinha Roy, A., Back, M. R., Khoury, S. F., Schneeberger, E. W., Back, L. H., Velury, V. V., Millard, R. W., & Banerjee, R. K. (2008). Functional and anatomical diagnosis of coronary artery stenoses. The Journal of Surgical Research, 150, 24–33.
Kroma, G., Lopera, J., Cura, M., Suri, R., El-Merhi, F., & Reading, J. (2009). Transjugular intrahepatic portosystemic shunt flow reduction with adjustable polytetrafluoroethylene-covered balloon-expandable stents. Journal of Vascular and Interventional Radiology JVIR, 20, 981–986.
Young, D., Cholvin, N., & Roth, A. (1975). Pressure drop across artificially induced stenoses in the femoral arteries of dogs. Circulation Research, 36, 735–743.
McMahon, M., Brown, B., Cukingnan, R., Rolett, E., Bolson, E., Frimer, M., & Dodge, H. (1979). Quantitative coronary angiography: measurement of the “critical” stenosis in patients with unstable angina and single-vessel disease without collaterals. Circulation, 60, 106–113.
Eklöf, B. (1970). Critical stenosis of the carotid artery in the dog. Scandinavian Journal of Clinical and Laboratory Investigation, 25, 349.
Gould, K., Kirkeeide, R., & Buchi, M. (1990). Coronary flow reserve as a physiologic measure of stenosis severity. Journal of the American College of Cardiology, 15, 459–474.
Waksman, R., Legutko, J., Singh, J., Orlando, Q., Marso, S., Schloss, T., Tugaoen, J., DeVries, J., Palmer, N., Haude, M., Swymelar, S., & Torguson, R. (2013). First: fractional flow reserve and intravascular ultrasound relationship study. Journal of the American College of Cardiology, 61, 917–923.
Kang, S.-J., Lee, J.-Y., Ahn, J.-M., Mintz, G. S., Kim, W.-J., Park, D.-W., Yun, S.-C., Lee, S.-W., Kim, Y.-H., Whan Lee, C., Park, S.-W., & Park, S.-J. (2011). Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity/clinical perspective. Circulation. Cardiovascular Interventions, 4, 65–71.
Koo, B.-K., Yang, H.-M., Doh, J.-H., Choe, H., Lee, S.-Y., Yoon, C.-H., Cho, Y.-K., Nam, C.-W., Hur, S.-H., Lim, H.-S., Yoon, M.-H., Park, K.-W., Na, S.-H., Youn, T.-J., Chung, W.-Y., Ma, S., Park, S.-K., Kim, H.-S., & Tahk, S.-J. (2011). Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. Journal of the American College of Cardiology: Cardiovascular Interventions, 4, 803–811.
Ben-Dor, I., Torguson, R., Gaglia, M. A., Jr., Gonzalez, M. A., Maluenda, G., Bui, A. B., Xue, Z., Satler, L. F., Suddath, W. O., Lindsay, J., Pichard, A. D., & Waksman, R. (2011). Correlation between fractional flow reserve and intravascular ultrasound lumen area in intermediate coronary artery stenosis. EuroIntervention, 7, 225–233.
Goldstein, R. A., Kirkeeide, R. L., Demer, L. L., Merhige, M., Nishikawa, A., Smalling, R. W., Mullani, N. A., & Gould, K. L. (1987). Relation between geometric dimensions of coronary artery stenoses and myocardial perfusion reserve in man. The Journal of Clinical Investigation, 79, 1473–1478.
Brosh, D., Higano, S. T., Lennon, R. J., Holmes, D. R., Jr., & Lerman, A. (2005). Effect of lesion length on fractional flow reserve in intermediate coronary lesions. American Heart Journal, 150, 338–343.
Kristensen, T. S., Engstrøm, T., Kelbæk, H., von der Recke, P., Nielsen, M. B., & Kofoed, K. F. (2010). Correlation between coronary computed tomographic angiography and fractional flow reserve. International Journal of Cardiology, 144, 200–205.
Acknowledgments
The authors would like to acknowledge the support of Amelie Bouchard, Martin Laflamme, Louis George Guy, and the Accellab team for their support in conducting the animal experiments.
Sources of funding
The authors acknowledge support from the Wellcome Trust Foundation for this study. Dr. Nijjer (G1100443) and Dr. Sen (G1000357) are Medical Research Council fellows. Dr. Petraco (FS/11/46/28861), Dr. JE Davies (FS/05/006), and Dr. Francis (FS 10/038) are British Heart Foundation fellows.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foin, N., Sen, S., Petraco, R. et al. Method for Percutaneously Introducing, and Removing, Anatomical Stenosis of Predetermined Severity In Vivo: The “Stenotic Stent”. J. of Cardiovasc. Trans. Res. 6, 640–648 (2013). https://doi.org/10.1007/s12265-013-9476-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9476-x