Abstract
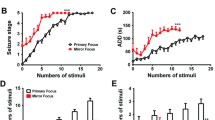
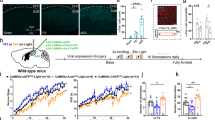
Secondary epileptogenesis is characterized by increased epileptic susceptibility and a tendency to generate epileptiform activities outside the primary focus. It is one of the major resultants of pharmacoresistance and failure of surgical outcomes in epilepsy, but still lacks effective treatments. Here, we aimed to test the effects of low-frequency stimulation (LFS) at the subiculum for secondary epileptogenesis in a mouse model. Here, secondary epileptogenesis was simulated at regions both contralateral and ipsilateral to the primary focus by applying successive kindling stimuli. Mice kindled at the right CA3 showed higher seizure susceptibilities at both the contralateral CA3 and the ipsilateral entorhinal cortex and had accelerated kindling processes compared with naive mice. LFS at the ipsilateral subiculum during the primary kindling progress at the right CA3 effectively prevented secondary epileptogenesis at both the contralateral CA3 and the ipsilateral entorhinal cortex, characterized by decreased seizure susceptibilities and a retarded kindling process at those secondary foci. Only application along with the primary epileptogenesis was effective. Notably, the effects of LFS on secondary epileptogenesis were associated with its inhibitory effect at the secondary focus through interfering with the enhancement of synaptic connections between the primary and secondary foci. These results imply that LFS at the subiculum is an effective preventive strategy for extensive secondary epileptogenesis in temporal lobe epilepsy and present the subiculum as a target with potential translational importance.






Similar content being viewed by others
References
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55: 475–482.
Goldensohn ES. The relevance of secondary epileptogenesis to the treatment of epilepsy: Kindling and the mirror focus. Epilepsia 1984, 25: S156–S173.
Shen Y, Gong Y, Ruan Y, Chen Z, Xu C. Secondary epileptogenesis: Common to see, but possible to treat? Front Neurol 2021, 12: 747372.
Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59: 2179–2193.
Xu C, Gong Y, Wang Y, Chen Z. New advances in pharmacoresistant epilepsy towards precise management-from prognosis to treatments. Pharmacol Ther 2022, 233: 108026.
Xu C, Wang Y, Zhang S, Nao J, Liu Y, Wang Y, et al. Subicular pyramidal neurons gate drug resistance in temporal lobe epilepsy. Ann Neurol 2019, 86: 626–640.
Paschen E, Elgueta C, Heining K, Vieira DM, Kleis P, Orcinha C, et al. Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy. Elife 2020, 9: e54518.
Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia 2018, 59: 273–290.
Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol 2004, 3: 111–118.
Fisher RS. Deep brain stimulation of thalamus for epilepsy. Neurobiol Dis 2023, 179: 106045.
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010, 51: 899–908.
Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: State-of-the-art approved therapies. Lancet Neurol 2021, 20: 1038–1047.
Moss J, Ryder T, Aziz TZ, Graeber MB, Bain PG. Electron microscopy of tissue adherent to explanted electrodes in dystonia and Parkinson’s disease. Brain 2004, 127: 2755–2763.
Feddersen B, Vercueil L, Noachtar S, David O, Depaulis A, Deransart C. Controlling seizures is not controlling epilepsy: A parametric study of deep brain stimulation for epilepsy. Neurobiol Dis 2007, 27: 292–300.
Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol 2013, 74: 223–231.
Koubeissi MZ, Joshi S, Eid A, Emami M, Jaafar N, Syed T, et al. Low-frequency stimulation of a fiber tract in bilateral temporal lobe epilepsy. Epilepsy Behav 2022, 130: 108667.
Wang Y, Liang J, Xu C, Wang Y, Kuang Y, Xu Z, et al. Low-frequency stimulation in anterior nucleus of thalamus alleviates kainate-induced chronic epilepsy and modulates the hippocampal EEG rhythm. Exp Neurol 2016, 276: 22–30.
Toprani S, Durand DM. Long-lasting hyperpolarization underlies seizure reduction by low frequency deep brain electrical stimulation. J Physiol 2013, 591: 5765–5790.
Kuang Y, Xu C, Zhang Y, Wang Y, Wu X, Wang Y, et al. Low-frequency stimulation of the primary focus retards positive transfer of secondary focus. Sci Rep 2017, 7: 345.
Wu DC, Xu ZH, Wang S, Fang Q, Hu DQ, Li Q, et al. Time-dependent effect of low-frequency stimulation on amygdaloid-kindling seizures in rats. Neurobiol Dis 2008, 31: 74–79.
O’Mara SM, Commins S, Anderson M, Gigg J. The subiculum: A review of form, physiology and function. Prog Neurobiol 2001, 64: 129–155.
Fei F, Wang X, Wang Y, Chen Z. Dissecting the role of subiculum in epilepsy: Research update and translational potential. Prog Neurobiol 2021, 201: 102029.
Lévesque M, Avoli M. The subiculum and its role in focal epileptic disorders. Rev Neurosci 2021, 32: 249–273.
Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci 2007, 27: 9866–9873.
Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron 2017, 95: 1221.
Xu C, Zhang S, Gong Y, Nao J, Shen Y, Tan B, et al. Subicular caspase-1 contributes to pharmacoresistance in temporal lobe epilepsy. Ann Neurol 2021, 90: 377–390.
Fei F, Wang X, Xu C, Shi J, Gong Y, Cheng H, et al. Discrete subicular circuits control generalization of hippocampal seizures. Nat Commun 2022, 13: 5010.
Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol 2005, 566: 885–900.
Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, et al. Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis 2012, 48: 20–26.
Ruan Y, Xu C, Lan J, Nao J, Zhang S, Fan F, et al. Low-frequency stimulation at the subiculum is anti-convulsant and anti-drug-resistant in a mouse model of lamotrigine-resistant temporal lobe epilepsy. Neurosci Bull 2020, 36: 654–658.
Vázquez-Barrón D, Cuéllar-Herrera M, Velasco F, Velasco AL. Electrical stimulation of subiculum for the treatment of refractory mesial temporal lobe epilepsy with hippocampal sclerosis: A 2-year follow-up study. Stereotact Funct Neurosurg 2021, 99: 40–47.
Bondallaz P, Boëx C, Rossetti AO, Foletti G, Spinelli L, Vulliemoz S, et al. Electrode location and clinical outcome in hippocampal electrical stimulation for mesial temporal lobe epilepsy. Seizure 2013, 22: 390–395.
Chen B, Xu C, Wang Y, Lin W, Wang Y, Chen L, et al. A disinhibitory nigra-parafascicular pathway amplifies seizure in temporal lobe epilepsy. Nat Commun 2020, 11: 923.
Xu CL, Nao JZ, Shen YJ, Gong YW, Tan B, Zhang S, et al. Long-term music adjuvant therapy enhances the efficacy of sub-dose antiepileptic drugs in temporal lobe epilepsy. CNS Neurosci Ther 2022, 28: 206–217.
Paxinos G, Watson G (1998) The Rat Brain in Stereotaxic Coordinates, 4th edn. Academic Press, San Diego, pp 96–101.
Wang Y, Wang Y, Xu C, Wang S, Tan N, Chen C, et al. Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol Psychiatry 2020, 87: 843–856.
Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 1972, 32: 281–294.
Löscher W. Animal models of seizures and epilepsy: Past, present, and future role for the discovery of antiseizure drugs. Neurochem Res 2017, 42: 1873–1888.
Sutula T, Harrison C, Steward O. Chronic epileptogenesis induced by kindling of the entorhinal cortex: The role of the dentate gyrus. Brain Res 1986, 385: 291–299.
Spiller AE, Racine RJ. Transfer kindling between sites in the entorhinal cortex-perforant path-dentate gyrus system. Brain Res 1994, 635: 130–138.
Xu Z, Wang Y, Chen B, Xu C, Wu X, Wang Y, et al. Entorhinal principal neurons mediate brain-stimulation treatments for epilepsy. EBioMedicine 2016, 14: 148–160.
Xu C, Wang S, Wang Y, Lin K, Pan G, Xu Z, et al. A decrease of ripples precedes seizure onset in mesial temporal lobe epilepsy. Exp Neurol 2016, 284: 29–37.
Gerlei KZ, Brown CM, Sürmeli G, Nolan MF. Deep entorhinal cortex: From circuit organization to spatial cognition and memory. Trends Neurosci 2021, 44: 876–887.
Robinson GB. Kindling-induced potentiation of excitatory and inhibitory inputs to hippocampal dentate granule cells. II. Effects of the NMDA antagonist MK-801. Brain Res 1991, 562: 26–33.
Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 2004, 92: 600–608.
Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat Neurosci 2003, 6: 1079–1085.
Liu Y, Wang Y, Xu Z, Xu C, Ying X, Wang S, et al. Consecutive 15 min is necessary for focal low frequency stimulation to inhibit amygdaloid-kindling seizures in rats. Epilepsy Res 2013, 106: 47–53.
Sobayo T, Mogul DJ. Rapid onset of a kainate-induced mirror focus in rat hippocampus is mediated by contralateral AMPA receptors. Epilepsy Res 2013, 106: 35–46.
Cela E, McFarlan AR, Chung AJ, Wang T, Chierzi S, Murai KK, et al. An optogenetic kindling model of neocortical epilepsy. Sci Rep 2019, 9: 5236.
Ben-Ari Y, Dudek FE. Primary and secondary mechanisms of epileptogenesis in the temporal lobe: There is a before and an after. Epilepsy Curr 2010, 10: 118–125.
Eliashiv SD, Dewar S, Wainwright I, Engel J, Fried I. Long-term follow-up after temporal lobe resection for lesions associated with chronic seizures. Neurology 1997, 48: 1383–1388.
Gollwitzer S, Scott CA, Farrell F, Bell GS, de Tisi J, Walker MC, et al. The long-term course of temporal lobe epilepsy: From unilateral to bilateral interictal epileptiform discharges in repeated video-EEG monitorings. Epilepsy Behav 2017, 68: 17–21.
Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol 2014, 10: 261–270.
Doyle CA, Cullen WK, Rowan MJ, Anwyl R. Low-frequency stimulation induces homosynaptic depotentiation but not long-term depression of synaptic transmission in the adult anaesthetized and awake rat hippocampus in vivo. Neuroscience 1997, 77: 75–85.
Morrell F. Secondary epileptogenesis in man. Arch Neurol 1985, 42: 318–335.
Morrell F, deToledo-Morrell L. From mirror focus to secondary epileptogenesis in man: An historical review. Adv Neurol 1999, 81: 11–23.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (U21A20418 and 82173796); the Natural Science Foundation of Zhejiang Province (LD22H310003); and the Research Project of Zhejiang Chinese Medical University (2023JKZDZC04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, Y., Gong, Y., Da, X. et al. Low-frequency Stimulation at the Subiculum Prevents Extensive Secondary Epileptogenesis in Temporal Lobe Epilepsy. Neurosci. Bull. (2024). https://doi.org/10.1007/s12264-023-01173-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12264-023-01173-z