Abstract
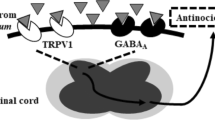
The present study was designed to investigate the effects of histamine on spontaneous neuropathic pain (NP) induced by peripheral axotomy. Rats and mice were subjected to complete transection of the left sciatic and saphenous nerves to induce spontaneous NP (the neuroma model). Rats were then treated with drugs once daily for 30 days (histidine and loratadine, i.p.) or 21 days (histamine, i.c.v.). Autotomy behavior was scored daily until day 50 post-operation (PO). On days 14 to 21 PO, some rats in the control group were subjected to single-fiber recording. Autotomy behavior was also monitored daily in histidine decarboxylase (the key enzyme for histamine synthesis) knockout (HDC-/-) and wild-type mice for 42 days. We found that both histidine (500 mg/kg) (a precursor of histamine that increases histamine levels in the tissues) and histamine (50 μg/5 μL) significantly suppressed autotomy behavior in rats. HDC-/- mice lacking endogenous histamine showed higher levels of autotomy than the wild-type. In addition, the analgesic effect of histidine was not antagonized by loratadine (a peripherally-acting H1 receptor antagonist), while loratadine alone significantly suppressed autotomy. Electrophysiological recording showed that ectopic spontaneous discharges from the neuroma were blocked by systemic diphenhydramine (an H1 receptor antagonist). Our results suggest that histamine plays an important role in spontaneous NP. It is likely that histamine in the central nervous system is analgesic, while in the periphery, via H1 receptors, it is algesic. This study justifies the avoidance of a histamine-rich diet and the use of peripherally-acting H1 receptor antagonists as well as agents that improve histamine action in the central nervous system in patients with spontaneous NP.
Similar content being viewed by others
References
Chong MS, Bajwa ZH. Diagnosis and treatment of neuropathic pain. J Pain Symptom Manage 2003, 25: S4–S11.
Dieleman JP, Kerklaan J, Huygen FJ, Bouma PA, Sturkenboom MC. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008, 137: 681–688.
Hall GC, Carroll D, Parry D, McQuay HJ. Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain 2006, 122: 156–162.
Farzin D, Asghari L, Nowrouzi M. Rodent antinociception following acute treatment with different histamine receptor agonists and antagonists. Pharmacol Biochem Behav 2002, 72: 751–760.
Kajihara Y, Murakami M, Imagawa T, Otsuguro K, Ito S, Ohta T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience 2010, 166: 292–304.
Tamaddonfard E, Rahimi S. Central effect of histamine and peripheral effect of histidine on the formalin-induced pain response in mice. Clin Exp Pharmacol Physiol 2004, 31: 518–522.
Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain 2003, 105: 467–479.
Huang L, Adachi N, Nagaro T, Liu K, Arai T. Histaminergic involvement in neuropathic pain produced by partial ligation of the sciatic nerve in rats. Reg Anesth Pain Med 2007, 32: 124–129.
Medhurst SJ, Collins SD, Billinton A, Bingham S, Dalziel RG, Brass A, et al. Novel histamine H3 receptor antagonists GSK189254 and GSK334429 are efficacious in surgicallyinduced and virally-induced rat models of neuropathic pain. Pain 2008, 138: 61–69.
Smith FM, Haskelberg H, Tracey DJ, Moalem-Taylor G. Role of histamine H3 and H4 receptors in mechanical hyperalgesia following peripheral nerve injury. Neuroimmunomodulation 2007, 14: 317–325.
Baron R, Schwarz K, Kleinert A, Schattschneider J, Wasner G. Histamine-induced itch converts into pain in neuropathic hyperalgesia. Neuroreport 2001, 12: 3475–3478.
Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol 2006, 198: 72–80.
Wall PD, Gutnick M. Ongoing activity in peripheral nerves: the physiology and pharmacology of impulses originating from a neuroma. Exp Neurol 1974, 43: 580–593.
Devor M. Sensory basis of autotomy in rats. Pain 1991, 45: 109–110.
Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol 2001, 429: 23–37.
Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett 2001, 502: 53–56.
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16: 109–110.
Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, et al. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain 1979, 7: 103–111.
Zhang SH, Blech-Hermoni Y, Faravelli L, Seltzer Z. Ralfinamide administered orally before hindpaw neurectomy or postoperatively provided long-lasting suppression of spontaneous neuropathic pain-related behavior in the rat. Pain 2008, 139: 293–305.
Raber P, Devor M. Social variables affect phenotype in the neuroma model of neuropathic pain. Pain 2002, 97: 139–150.
Barnett A, Iorio LC, Kreutner W, Tozzi S, Ahn HS, Gulbenkian A. Evaluation of the CNS properties of SCH 29851, a potential non-sedating antihistamine. Agents Actions 1984, 14: 590–597.
Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009, 196: 115–128.
Seltzer Z, Paran Y, Eisen A, Ginzburg R. Neuropathic pain behavior in rats depends on the afferent input from nerve-end neuroma including histamine-sensitive C-fibers. Neurosci Lett 1991, 128: 203–206.
Ji RR. Recent progress in understanding the mechanisms of pain and itch. Neurosci Bull 2012, 28: 89–90.
Magerl W, Westerman RA, Mohner B, Handwerker HO. Properties of transdermal histamine iontophoresis: differential effects of season, gender, and body region. J Invest Dermatol 1990, 94: 347–352.
Simone DA, Ngeow JY, Whitehouse J, Becerra-Cabal L, Putterman GJ, LaMotte RH. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosens Res 1987, 5: 81–92.
Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 2006, 124: 50–58.
Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol 2001, 81: 250–254.
Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci 1997, 17: 8003–8008.
Jochem J. Endogenous central histamine-induced reversal of critical hemorrhagic hypotension in rats: studies with Lhistidine. Shock 2003, 20: 332–337.
Khalilzadeh E, Tamaddonfard E, Farshid AA, Erfanparast A. Microinjection of histamine into the dentate gyrus produces antinociception in the formalin test in rats. Pharmacol Biochem Behav 2010, 97: 325–332.
Watanabe T, Yanai K. Studies on functional roles of the histaminergic neuron system by using pharmacological agents, knockout mice and positron emission tomography. Tohoku J Exp Med 2001, 195: 197–217.
Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci 2007, 28: 350–357.
Seltzer Z, Tal M, Sharav Y. Autotomy behavior in rats following peripheral deafferentation is suppressed by daily injections of amitriptyline, diazepam and saline. Pain 1989, 37: 245–250.
Author information
Authors and Affiliations
Corresponding authors
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, J., Lou, GD., Yue, JX. et al. Effects of histamine on spontaneous neuropathic pain induced by peripheral axotomy. Neurosci. Bull. 29, 261–269 (2013). https://doi.org/10.1007/s12264-013-1316-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-013-1316-0