Abstract
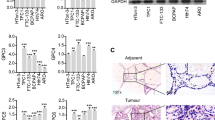
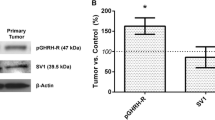
Neurotransmitter systems have recently been shown to be involved in multiple malignancies including breast, colon and prostate cancers. The role of neurotransmitters and neurotrophic factors has not yet been examined in thyroid cancer. To determine the possible involvement of neurotransmitter systems in thyroid carcinogenesis we characterized the patterns of gamma-aminobutyric acid (GABA) receptor expression in normal thyroid and thyroid tumors. We examined the expression patterns of the GABAergic system in 70 human thyroid tumor samples (13 follicular adenomas, 14 follicular carcinomas, 43 papillary carcinomas) and adjacent normal thyroid by immunohistochemistry. GABAergic system mRNA expression in thyroid cancer cell lines derived from primary (FTC133) and metastatic tumors (FTC236 and FTC238) was examined by real time PCR. Overall, GABA receptor expression is increased in tumors compared to normal thyroid tissue. Expression of GABAA receptor β2 was detected in the vasculature of normal thyroid and thyroid tumors but not in thyroid cancer cells. GABAA α2 was detected in metastatic-derived but not in primary-tumor derived cell lines. Expression levels of GABAB R2 and GABA receptor associated protein (GABARAP) are increased in adenomas and thyroid cancer suggesting their role in early stages of thyroid tumorigenesis. This study represents the first demonstration of GABA receptor expression in human thyroid tissue and suggests that the GABAergic system is involved in thyroid carcinogenesis.

Similar content being viewed by others
Abbreviations
- GABA:
-
Gamma-aminobutyric Acid
- GABARAP:
-
GABA receptor associated protein
- PCR:
-
Polymerase chain reaction
- FC:
-
Follicular carcinoma
- PC:
-
Papillary carcinoma
References
Ries LAG, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK (2008) SEER Cancer Statistics Review, 1975–2005. via http://seer.cancer.gov/csr/1975_2005/ Cited 04 December 2008
DeGroot LJ, Kaplan EL, McCormick M, Straus FH (1990) Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab 71:414–424
Ringel MD, Ladenson PW (2004) Controversies in the follow-up and management of well-differentiated thyroid cancer. Endocr Relat Cancer 11:97–116
Vasko V, Bauer AJ, Tuttle RM, Francis GL (2007) Papillary and follicular thyroid cancers in children. Endocr Dev 10:140–172
Hundahl SA, Fleming ID, Fremgen AM, Menck HR (1998) A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 83:2638–2648
Ain KB, Egorin MJ, DeSimone PA (2000) Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative anaplastic thyroid cancer health intervention trials (CATCHIT) group. Thyroid 10:587–594
Entschladen F, Palm D, Lang K, TLt D, Zaenker KS (2006) Neoneurogenesis: tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med Hypotheses 67:33–35
Palm D, Entschladen F (2007) Neoneurogenesis and the neuro-neoplastic synapse. Prog Exp Tumor Res 39:91–98
Lang K, TLt D, Zaenker KS, Entschladen F (2006) Inhibitors for metastasis development. Recent Patents Anticancer Drug Discov 1:69–80
Macdonald RL, Olsen RW (1994) GABAA receptor channels. Annu Rev Neurosci 17:569–602
Akinci MK, Schofield PR (1999) Widespread expression of GABA(A) receptor subunits in peripheral tissues. Neurosci Res 35:145–153
Ong J, Kerr DI (1990) GABA-receptors in peripheral tissues. Life Sci 46:1489–1501
Wiens SC, Trudeau VL (2006) Thyroid hormone and gamma-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comp Biochem Physiol A Mol Integr Physiol 144:332–344
Gebauer H (1981) GABA transport in the rat thyroid. Naunyn Schmiedebergs Arch Pharmacol 317:61–66
Gebauer H, Pabst MA (1981) Autoradiographic localization of 3H-GABA uptake in the thyroid gland of the rat. Cell Tissue Res 220:873–879
Martin JV, Williams DB, Fitzgerald RM, Im HK, Vonvoigtlander PF (1996) Thyroid hormonal modulation of the binding and activity of the GABAA receptor complex of brain. Neuroscience 73:705–713
Mason GA, Walker CH, Prange AJ Jr, Bondy SC (1987) GABA uptake is inhibited by thyroid hormones: implications for depression. Psychoneuroendocrinology 12:53–59
Ahren B (1989) GABA inhibits thyroid hormone secretion in the mouse. Thyroidology 1:105–108
Jiang Y, Harlocker SL, Molesh DA, Dillon DC, Stolk JA, Houghton RL et al (2002) Discovery of differentially expressed genes in human breast cancer using subtracted cDNA libraries and cDNA microarrays. Oncogene 21:2270–2282
Kleinrok Z, Matuszek M, Jesipowicz J, Matuszek B, Opolski A, Radzikowski C (1998) GABA content and GAD activity in colon tumors taken from patients with colon cancer or from xenografted human colon cancer cells growing as s.c. tumors in athymic nu/nu mice. J Physiol Pharmacol 49:303–310
Matuszek M, Jesipowicz M, Kleinrok Z (2001) GABA content and GAD activity in gastric cancer. Med Sci Monit 7:377–381
Mazurkiewicz M, Opolski A, Wietrzyk J, Radzikowski C, Kleinrok Z (1999) GABA level and GAD activity in human and mouse normal and neoplastic mammary gland. J Exp Clin Cancer Res 18:247–253
Nicholson-Guthrie CS, Guthrie GD, Sutton GP, Baenziger JC (2001) Urine GABA levels in ovarian cancer patients: elevated GABA in malignancy. Cancer Lett 162:27–30
Opolski A, Mazurkiewicz M, Wietrzyk J, Kleinrok Z, Radzikowski C (2000) The role of GABA-ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. J Exp Clin Cancer Res 19:383–390
Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S et al (2007) Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA 104:2803–2808
Hemelaar J, Lelyveld VS, Kessler BM, Ploegh HL (2003) A single protease, Apg4B, is specific for the autophagy-related ubiquitin-like proteins GATE-16, MAP1-LC3, GABARAP, and Apg8L. J Biol Chem 278:51841–51850
Sou YS, Tanida I, Komatsu M, Ueno T, Kominami E (2006) Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J Biol Chem 281:3017–3024
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36:2503–2518
Disclaimer
The opinions or assertions contained herein are the personal views of the authors and are not to be construed as official or to reflect the opinions of the Uniformed Services University of the Health Sciences, the Department of the Army, or the Department of Defense.
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roberts, S.S., Mendonça-Torres, M.C., Jensen, K. et al. GABA Receptor Expression in Benign and Malignant Thyroid Tumors. Pathol. Oncol. Res. 15, 645–650 (2009). https://doi.org/10.1007/s12253-009-9165-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-009-9165-x