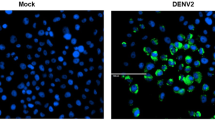
Abstract
Dengue virus (DENV) is an arthropod-borne viral pathogen and a global health burden. Knowledge of the DENV-host interactions that mediate virus pathogenicity remains limited. Host lipid metabolism is hijacked by DENV for virus replication in which lipid droplets (LDs) play a key role during the virus lifecycle. In this study, we reveal a novel role for phosphatase and tensin homolog deleted on chromosome 10 (PTEN) in LDs-mediated DENV infection. We demonstrate that PTEN expression is downregulated upon DENV infection through post-transcriptional regulation and, in turn, PTEN overexpression enhances DENV replication. PTEN lipid phosphatase activity was found to decrease cellular LDs area and number through Akt/FoxO1/Maf1 signaling, which, together with autophagy, enhanced DENV replication and virus production. We therefore provide mechanistic insight into the interaction between lipid metabolism and the DENV replication cycle.






Similar content being viewed by others
References
Aguiar M, Stollenwerk N, Halstead SB (2016) The impact of the newly licensed dengue vaccine in endemic countries. PLoS Negl Trop Dis 10:e0005179
Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, Brechot C (1997) Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci U S A 94:1200–1205
Bersuker K, Olzmann JA (2017) Establishing the lipid droplet proteome: mechanisms of lipid droplet protein targeting and degradation. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1166–1177
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496:504–507
Carvalho FA, Carneiro FA, Martins IC, Assuncao-Miranda I, Faustino AF, Pereira RM, Bozza PT, Castanho MARB, Mohana-Borges R, Da Poian AT, Santos NC (2012) Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface proteins. J Virol 86:2096–2108
Clement S, Peyrou M, Sanchez-Pareja A, Bourgoin L, Ramadori P, Suter D, Vinciguerra M, Guilloux K, Pascarella S, Rubbia-Brandt L, Negro F, Foti M (2011) Down-regulation of phosphatase and tensin homolog by hepatitis C virus core 3a in hepatocytes triggers the formation of large lipid droplets. Hepatology 54:38–49
Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R (2012) Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol 86:7637–7649
Cucunawangsih, Lugito NPH (2017) Trends of dengue disease epidemiology. Virology (Auckl) 8:1178122X17695836
Dalugama C, Gawarammana IB (2018) Lessons learnt from managing a case of dengue hemorrhagic fever complicated with acute liver failure and acute kidney injury: a case report. J Med Case Rep 12:215
Davidson L, Maccario H, Perera NM, Yang X, Spinelli L, Tibarewal P, Glancy B, Gray A, Weijer CJ, Downes CP, Leslie NR (2010) Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29:687–697
Delbruck M (1940) The growth of bacteriophage and lysis of the host. J Gen Physiol 23:643–660
Deng X, Zhang W, O-Sullivan I, Williams JB, Dong Q, Park EA, Raghow R, Unterman TG, Elam MB (2012) FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J Biol Chem 287:20132–20143
Ding Y, Zhang S, Yang L, Na H, Zhang P, Zhang H, Wang Y, Chen Y, Yu J, Huo C, Xu S, Garaiova M, Cong Y, Liu P (2013) Isolating lipid droplets from multiple species. Nat Protoc 8:43–51
Domitrovich AM, Felmlee DJ, Siddiqui A (2005) Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J Biol Chem 280:39802–39808
Gao TT, Qin ZL, Ren H, Zhao P, Qi ZT (2015) Inhibition of IRS-1 by hepatitis C virus infection leads to insulin resistance in a PTEN-dependent manner. Virol J 12:12
Gould EA, Solomon T (2008) Pathogenic flaviviruses. Lancet 371:500–509
Halstead SB (2003) Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467
He L, Hou X, Kanel G, Zeng N, Galicia V, Wang Y, Yang J, Wu H, Birnbaum MJ, Stiles BL (2010) The critical role of AKT2 in hepatic steatosis induced by PTEN loss. Am J Pathol 176:2302–2308
Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G (2010) Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci U S A 107:17345–17350
Heaton NS, Randall G (2010) Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8:422–432
Iglesias NG, Mondotte JA, Byk LA, De Maio FA, Samsa MM, Alvarez C, Gamarnik AV (2015) Dengue virus uses a non-canonical function of the host GBF1-Arf-COPI system for capsid protein accumulation on lipid droplets. Traffic 16:962–977
Johnson DL, Stiles BL (2016) Maf1, a new PTEN target linking rna and lipid metabolism. Trends Endocrinol Metab 27:742–750
Johnson SS, Zhang C, Fromm J, Willis IM, Johnson DL (2007) Mammalian Maf1 is a negative regulator of transcription by all three nuclear RNA polymerases. Mol Cell 26:367–379
Khetarpal N, Khanna I (2016) Dengue fever: causes, complications, and vaccine strategies. J Immunol Res 2016:6803098
Kinkel AD, Fernyhough ME, Helterline DL, Vierck JL, Oberg KS, Vance TJ, Hausman GJ, Hill RA, Dodson MV (2004) Oil red-O stains non-adipogenic cells: a precautionary note. Cytotechnology 46:49–56
Li Z, Li J, Bi P, Lu Y, Burcham G, Elzey BD, Ratliff T, Konieczny SF, Ahmad N, Kuang S, Liu X (2014) Plk1 phosphorylation of PTEN causes a tumor-promoting metabolic state. Mol Cell Biol 34:3642–3661
Lin P, Chen X, Moktan H, Arrese EL, Duan L, Wang L, Soulages JL, Zhou DH (2014) Membrane attachment and structure models of lipid storage droplet protein 1. Biochim Biophys Acta 1838:874–881
Martins IC, Gomes-Neto F, Faustino AF, Carvalho FA, Carneiro FA, Bozza PT, Mohana-Borges R, Castanho MA, Almeida FC, Santos NC, Da Poian AT (2012) The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. Biochem J 444:405–415
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Murray NE, Quam MB, Wilder-Smith A (2013) Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 5:299–309
Mustafa MS, Rasotgi V, Jain S, Gupta V (2015) Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India 71:67–70
Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK (1998) The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA 95:13513–13518
Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK (1997) P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA 94:9052–9057
Naderali E, Khaki AA, Rad JS, Ali-Hemmati A, Rahmati M, Charoudeh HN (2018) Regulation and modulation of PTEN activity. Mol Biol Rep 45:2869–2881
Ortega-Molina A, Serrano M (2013) PTEN in cancer, metabolism, and aging. Trends Endocrinol Metab 24:184–189
Palian BM, Rohira AD, Johnson SAS, He L, Zheng N, Dubeau L, Stiles BL, Johnson DL (2014) Maf1 is a novel target of PTEN and PI3K signaling that negatively regulates oncogenesis and lipid metabolism. Plos Genet 10:e1004789
Peng H, Liu B, Yves TD, He Y, Wang S, Tang H, Ren H, Zhao P, Qi Z, Qin Z (2018) Zika virus induces autophagy in human umbilical vein endothelial cells. Viruses 10:259
Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ (2012) Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog 8:e1002584
Peyrou M, Clement S, Maier C, Bourgoin L, Branche E, Conzelmann S, Kaddai V, Foti M, Negro F (2013) PTEN protein phosphatase activity regulates hepatitis C virus secretion through modulation of cholesterol metabolism. J Hepatol 59:420–426
Pluta K, Lefebvre O, Martin NC, Smagowicz WJ, Stanford DR, Ellis SR, Hopper AK, Sentenac A, Boguta M (2001) Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae. Mol Cell Biol 21:5031–5040
Poh MK, Shui G, Xie X, Shi PY, Wenk MR, Gu F (2012) U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Res 93:191–198
Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8:224–236
Qin ZL, Ju HP, Gao TT, Wang WB, Ren H, Zhao P, Qi ZT (2015) Two conserved histidines (His490 and His621) on the E2 glycoprotein of hepatitis C virus are critical for CD81-mediated cell entry. J Gen Virol 96:1389–1399
Qin ZL, Ju HP, Wang WB, Ren H, Guan M, Zhao P, Qi ZT (2013) The Arg719 residue at the C-terminal end of the stem region in hepatitis C virus JFH-1 E2 glycoprotein promotes viral infection. Virus Res 172:1–8
Qin ZL, Zhao P, Cao MM, Qi ZT (2007) siRNAs targeting terminal sequences of the SARS-associated coronavirus membrane gene inhibit M protein expression through degradation of M mRNA. J Virol Methods 145:146–154
Qin ZL, Zhao P, Zhang XL, Yu JG, Cao MM, Zhao LJ, Luan J, Qi ZT (2004) Silencing of SARS-CoV spike gene by small interfering RNA in HEK 293T cells. Biochem Biophys Res Commun 324:1186–1193
Randall G (2018) Lipid droplet metabolism during dengue virus infection. Trends Microbiol 26:640–642
Rastogi M, Singh SK (2019) Modulation of type-I interferon response by hsa-miR-374b-5p during japanese encephalitis virus infection in human microglial cells. Front Cell Infect Microbiol 9:291
Rodenhuis-Zybert IA, Wilschut J, Smit JM (2010) Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci 67:2773–2786
Roh MH, Yassin Y, Miron A, Mehra KK, Mehrad M, Monte NM, Mutter GL, Nucci MR, Ning G, McKeon FD, Hirsch MS, Wa X, Crum CP (2010) High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod Pathol 23:1316–1324
Samanta J, Sharma V (2015) Dengue and its effects on liver. World J Clin Cases 3:125–131
Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV (2009) Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog 5:e1000632
Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13:283–296
Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, Wu H (2004) Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc Natl Acad Sci U S A 101:2082–2087
Tang WC, Lin RJ, Liao CL, Lin YL (2014) Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol 88:6793–6804
Upadhya R, Lee J, Willis IM (2002) Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell 10:1489–1494
Walther TC, Farese RV Jr (2012) Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 81:687–714
Wang CW (2016) Lipid droplets, lipophagy, and beyond. Biochim Biophys Acta 1861:793–805
Waris G, Felmlee DJ, Negro F, Siddiqui A (2007) Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J Virol 81:8122–8130
Yu N, Puckett S, Antinozzi PA, Cramer SD, Lyles DS (2015) Changes in susceptibility to oncolytic vesicular stomatitis virus during progression of prostate cancer. J Virol 89:5250–5263
Yung CF, Lee KS, Thein TL, Tan LK, Gan VC, Wong JGX, Lye DC, Ng LC, Leo YS (2015) Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, singapore. Am J Trop Med Hyg 92:999–1005
Zhang J, Lan Y, Sanyal S (2017) Modulation of lipid droplet metabolism-a potential target for therapeutic intervention in flaviviridae infections. Front Microbiol 8:2286
Zhang JS, Lan Y, Li MY, Lamers MM, Fusade-Boyer M, Klemm E, Thiele C, Ashour J, Sanyal S (2018) Flaviviruses exploit the lipid droplet protein AUP1 to trigger lipophagy and drive virus production. Cell Host Microbe 23:819–831
Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG (2006) FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281:10105–10117
Acknowledgements
This study was funded by the National Natural Science Foundation of China (81171564), the National Key Research and Development Program of China (2016YFC1200400) and the National S&T Major Project for Infectious Diseases Control (2017ZX10304403). The authors thank Yingfeng Lei (Fourth Military Medical University, China) for providing us polyclonal anti-DENV-2 antibodies for immunofluorescence assay.
Author information
Authors and Affiliations
Contributions
QZT and QZL designed the experiments. LB, GTT, FXY, XZH carried out the experiments. QZL and LB analyzed the data and wrote the paper. All the authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, B., Gao, TT., Fu, XY. et al. PTEN Lipid Phosphatase Activity Enhances Dengue Virus Production through Akt/FoxO1/Maf1 Signaling. Virol. Sin. 36, 412–423 (2021). https://doi.org/10.1007/s12250-020-00291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-020-00291-6