Abstract
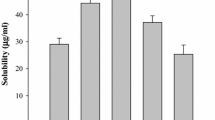
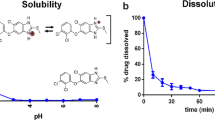
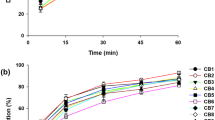
The objective of the current investigation was to enhance the solubility and dissolution rate of loratadine using solid dispersions (SDs) with Gelucire 50/13. SDs of loratadine using Gelucire 50/13 as carrier were prepared by the solvent evaporation method, characterized for drug content, dissolution behavior, and physicochemical characteristics by differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM) studies. At 10 % concentration of Gelucire 50/13, the increase in solubility was around 100-fold compared with pure drug. The solubility of loratadine in the presence of Gelucire 50/13 in water showed linear increase with increasing concentrations of Gelucire indicating AL-type solubility diagrams. The mean dissolution time (MDT) of loratadine decreased after preparation of SDs with Gelucire 50/13 indicating increased dissolution rate. FTIR studies showed the stability of loratadine and the absence of a well-defined interaction. DSC and XRD studies revealed the amorphous state of loratadine in SDs which was further confirmed from SEM. From the dissolution parameters, it is evident that the solubility and dissolution rate of loratadine was enhanced by SDs with Gelucire 50/13.







Similar content being viewed by others
References
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutical drug classification: correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Ghorab MK, Adeyeye MC. Enhancement of ibuprofen, dissolution via wet granulation with beta-cyclodextrin. Pharm Dev Technol. 2001;6:305–14.
Nokhodchi A, Javadzadeh Y, Siahi-Shadbad MR, Jalali BM. The effect of type and concentration of vehicles on the dissolution rate of poorly soluble drug (indomethacin) from liquisolid compacts. J Pharm Pharm Sci. 2005;8:18–25.
Kim CK, Cho YJ, Gao ZG. Preparation and evaluation of biphenyl dimethyl dicarboxylate microemulsions for oral delivery. J Control Release. 2001;70:149–55.
Elaine ML, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20.
Teofilo V, Bruno S, Paulo C. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12:1068–75.
Kai T, Akiyama Y, Nomura S, Sato M. Oral absorption improvement of poorly soluble drug using solid dispersion technique. Chem Pharm Bull. 1996;44:568–71.
Khan GM, Zhu JB. Preparation, characterization, and dissolution studies of ibuprofen solid dispersions using polyethylene glycol (PEG), talc, and PEG-talc as dispersion carriers. Drug Dev Ind Pharm. 1998;24:455–62.
Kearney AS, Gabriel DL, Mehta SC, Radebaugh GW. Effect of polyvinyl pyrrolidone on the crystallinity and dissolution rate of solid dispersions of the anti-inflammatory Ci-987. Int J Pharm. 1994;104:169–74.
Okonogi S, Oguchi T, Yonemochi E, Puttipipatkhachorn S, Yamamoto K. Improved dissolution of ofloxacin via solid dispersion. Int J Pharm. 1997;156:175–80.
Montousse C, Pruvost M, Rodriguez F, Brossard C. Extrusion-spheronization manufacture of Gelucire matrix beads. Drug Dev Ind Pharm. 1999;25:75–80.
Khan N, Craig DQM. The influence of drug incorporation on the structure and release properties of solid dispersions in the lipid matrices. J Control Release. 2003;93:355–68.
Choy YW, Khan N, Yuen KH. Significance of lipid matrix aging on in vitro release and in vivo bioavailability. Int J Pharm. 2005;299:55–64.
Perissutti B, Rubessa F, Princivalle F. Solid dispersions of carbamazepine with Gelucire 44/14 and 50/13. STP Pharm Sci. 2000;10:479–84.
Shimpi SL, Chauhan B, Mahadik KR, Paradkar A. Stabilization and improved in vivo performance of amorphous Etoricoxib using Gelucire 50/13. Pharm Res. 2005;10:1727–35.
Potluri RHK, Bandari S, Jukanti R, Veerareddy PR. Solubility enhancement and physicochemical characterization of carvedilol solid dispersion with Gelucire 50/13. Arch Pharm Res. 2011;34:51–7.
Ter Laak AM, Tsai RS, Donne-Op den Kelder GM, Carrupt PA, Testa B, Timmerman H. Lipophilicity and bonding capacity of H1-antihistaminic agents in relation to their central sedative side effects. Eur J Pharm Sci. 1994;2:373–84.
Khan MZI, Rausl D, Zanoski R, Zidar S, Mikulcic JH, Krizmanic L, et al. Classification of loratadine based on the biopharmaceutics drug classification concept and possible in vitro–in vivo correlation. Biol Pharm Bull. 2004;27:1630–5.
Sehgal A, Srivastava J, Arora VK. Nasal pharmaceutical compositions of loratadine. Patent no. WO 2004082589A2, 2004.
Abdel-Rahman SI, Ahmad SM, Samy IM, Badawy AM. Interactions of loratadine with cyclodextrins. Ethiop Pharm J. 1999;17:1–19.
Omar L, El-Barghouthi MI, Masoud NA, Abdoh AA, Al Omari MM, Zughul MB, et al. Inclusion complexation of loratadine with natural and modified cyclodextrins: Phase solubility and thermodynamic studies. J Solution Chem. 2007;36:605–16.
Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9.
Barzegar-Jalali M, Maleki N, Garjani A, Khandar AA, Haji-Hosseinloo M, Jabbari R, et al. Enhancement of dissolution rate and anti-inflammatory effects of piroxicam using solvent deposition technique. Drug Dev Ind Pharm. 2002;28:681–6.
Higuchi J, Connors K. Phase solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212.
Zerrouk N, Chemtob C, Arnaud P, Toscani S, Dugue J. In vitro and in vivo evaluation of carbamazepine-PEG 6000 solid dispersions. Int J Pharm. 2001;225:49–62.
Serajuddin ATM. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88:1058–66.
Tang L, Khan SU, Muhammad NA. Evaluation and selection of bio-relevant dissolution media for a poorly water soluble new chemical entity. Pharm Dev Technol. 2001;6:531–40.
Horter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastro intestinal tract. Adv Drug Deliv Rev. 1997;25:3–14.
Damian F, Blaton N, Naesens L, Balzarini J, Kinget R, Augustijns P, et al. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci. 2000;10:311–22.
Hancock BC, Zographi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86:1–12.
Okonogi S, Yonemochi E, Oguchi T, Puttipipatkhachorn S, Yamamoto K. Enhanced dissolution of ursodeoxycholic acid from the solid dispersion. Drug Dev Ind Pharm. 1997;23:1115–21.
Shin SC, Kim J. Physicochemical characterization of solid dispersion of furosemide with TPGS. Int J Pharm. 2003;251:79–84.
Acknowledgments
The authors acknowledge Gattefosse, France, for gift sample of Gelucire 50/13. The authors also thank Mr. T. Jaypal Reddy, Correspondent St. Peter’s Institute of Pharmaceutical Sciences, Hanamkonda, for providing facilities.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bandari, S., Jadav, S., Eedara, B.B. et al. Enhancement of Solubility and Dissolution Rate of Loratadine with Gelucire 50/13. J Pharm Innov 9, 141–149 (2014). https://doi.org/10.1007/s12247-014-9181-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-014-9181-6