Abstract
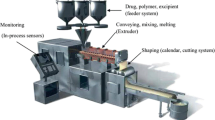
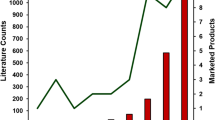
Drug and polymer mixing status in amorphous solid dispersions, an important aspect with regard to the physical stability and in vivo performance of such systems, was evaluated in this report with two case studies. In the first case study, the mixing between the drug and the polymer in an amorphous solid dispersion was assessed at both particulate and bulk levels to ensure that a homogeneous solid dispersion was obtained. In the second study, drug–polymer distribution evaluation in amorphous solid dispersions facilitated the selection of an optimal drug loading and a robust manufacturing process at the early stage of formulation development. Through these two case studies, it is suggested that establishing a multi-faceted characterization approach for amorphous solid dispersions is key to achieve a better understanding of these complex systems and successful delivery of stable and efficacious amorphous formulations.










Similar content being viewed by others
References
Engers D, Teng J, Jimenez-Novoa J, Gent P, Hossack S, Campbell C, et al. A solid-state approach to enable early development compounds: selection and animal bioavailability studies of an itraconazole amorphous solid dispersion. J Pharm Sci. 2010;99(9):3901–22.
Kennedy M, Hu J, Gao P, Li L, Ali-Reynolds A, Chal B, et al. Enhanced bioavailability of a poorly soluble VR1 antagonist using an amorphous solid dispersion approach: a case study. Mol Pharm. 2008;5(6):981–93.
Onoue S, Sato H, Ogawa K, Kawabata Y, Mizumoto T, Yuminoki K, et al. Improved dissolution and pharmacokinetic behavior of cyclosporine A using high-energy amorphous solid dispersion approach. Int J Pharm. 2010;399(1–2):94–101.
Serajuddin ATM. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci Us. 1999;88(10):1058–66.
Vranic E. Amorphous pharmaceutical solids. Bosn J Basic Med Sci. 2004;4(3):35–9.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42.
Leane MM, Sinclair W, Qian F, Haddadin R, Brown A, Tobyn M, et al. Formulation and process design for a solid dosage form containing a spray-dried amorphous dispersion of ibipinabant. Pharm Dev Technol. 2012;18(2):359–66.
Takeuchi H, Nagira S, Yamamoto H, Kawashima Y. Solid dispersion particles of amorphous indomethacin with fine porous silica particles by using spray-drying method. Int J Pharm. 2005;293(1–2):155–64.
Dong Z, Chatterji A, Sandhu H, Choi DS, Chokshi H, Shah N. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int J Pharm. 2008;355(1–2):141–9.
Chokshi RJ, Sandhu HK, Iyer RM, Shah NH, Malick AW, Zia H. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion processs as a means to manufacture solid dispersion/solution. J Pharm Sci Us. 2005;94(11):2463–74.
Lakshman JP, Cao Y, Kowalski J, Serajuddin AT. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol Pharm. 2008;5(6):994–1002.
Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm Res. 2012;29(3):806–17.
Hancock BC, Shamblin SL, Zografi G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm Res. 1995;12(6):799–806.
Yoshioka M, Hancock BC, Zografi G. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J Pharm Sci. 1995;84(8):983–6.
Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14(12):1691–8.
Matsumoto T, Zografi G. Physical properties of solid molecular dispersions of indomethacin with poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-vinylacetate) in relation to indomethacin crystallization. Pharm Res. 1999;16(11):1722–8.
Miyazaki T, Yoshioka S, Aso Y. Physical stability of amorphous acetanilide derivatives improved by polymer excipients. Chem Pharm Bull. 2006;54(8):1207–10.
Huang J, Wigent RJ, Schwartz JB. Drug–polymer interaction and its significance on the physical stability of nifedipine amorphous dispersion in microparticles of an ammonio methacrylate copolymer and ethylcellulose binary blend. J Pharm Sci Us. 2008;97(1):251–62.
Sugamura Y, Fujii M, Nakanishi S, Suzuki A, Shibata Y, Koizumi N, et al. Effect of particle size of drug on conversion of crystals to an amorphous state in a solid dispersion with crospovidone. Chem Pharm Bull (Tokyo). 2011;59(2):235–8.
Puri V, Dantuluri AK, Bansal AK. Investigation of atypical dissolution behavior of an encapsulated amorphous solid dispersion. J Pharm Sci. 2011;100(6):2460–8.
Qian F, Huang J, Zhu Q, Haddadin R, Gawel J, Garmise R, et al. Is a distinctive single Tg a reliable indicator for the homogeneity of amorphous solid dispersion? Int J Pharm. 2010;395(1–2):232–5.
Rumondor AC, Ivanisevic I, Bates S, Alonzo DE, Taylor LS. Evaluation of drug-polymer miscibility in amorphous solid dispersion systems. Pharm Res. 2009;26(11):2523–34.
Yoo SU, Krill SL, Wang Z, Telang C. Miscibility/stability considerations in binary solid dispersion systems composed of functional excipients towards the design of multi-component amorphous systems. J Pharm Sci. 2009;98(12):4711–23.
Onoue S, Sato H, Kawabata Y, Mizumoto T, Hashimoto N, Yamada S. In vitro and in vivo characterization on amorphous solid dispersion of cyclosporine A for inhalation therapy. J Control Release. 2009;138(1):16–23.
Baird JA, Taylor LS. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012;64(5):396–421.
Onoue S, Uchida A, Takahashi H, Seto Y, Kawabata Y, Ogawa K, et al. Development of high-energy amorphous solid dispersion of nanosized nobiletin, a citrus polymethoxylated flavone, with improved oral bioavailability. J Pharm Sci. 2011;100(9):3793–801.
Hasegawa S, Ke P, Buckton G. Determination of the structural relaxation at the surface of amorphous solid dispersion using inverse gas chromatography. J Pharm Sci. 2009;98(6):2133–9.
Hogan SE, Buckton G. The application of near infrared spectroscopy and dynamic vapor sorption to quantify low amorphous contents of crystalline lactose. Pharm Res. 2001;18(1):112–6.
Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm Res. 2006;23(10):2417–26.
Tao J, Sun Y, Zhang GGZ, Yu L. Solubility of small-molecule crystals in polymers: d-mannitol in PVP, indomethacin in PVP/VA, and nifedipine in PVP/VA. Pharm Res. 2009;26(4):855–64.
Qian F, Huang J, Hussain MA. Drug-polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99(7):2941–7.
Marsac PJ, Li T, Taylor LS. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2008;26(1):139–51.
Zhao Y, Inbar P, Chokshi HP, Malick AW, Choi DS. Prediction of the thermal phase diagram of amorphous solid dispersions by Flory-Huggins theory. J Pharm Sci. 2011;100(8):3196–207.
Lyon R, Lester D, Lewis E, Lee E, Yu L, Jefferson E, et al. Near-infrared spectral imaging for quality assurance of pharmaceutical products: analysis of tablets to assess powder blend homogeneity. AAPS Pharm Sci Tech. 2002;3:1–15.
Lewis EN, Carroll JE, Clarke F. NIR imaging: a near infrared view of pharmaceutical formulation analysis. NIR News. 2001;12:16–8.
Ellison CD, Ennis BJ, Hamad ML, Lyon RC. Measuring the distribution of density and tabletting force in pharmaceutical tablets by chemical imaging. J Pharm Biomed Anal. 2008;48(1):1–7.
Gowen AA, O’Donnell CP, Cullen PJ, Bell SEJ. Recent applications of chemical imaging to pharmaceutical process monitoring and quality control. Eur J Pharm Biopharm. 2008;69(1):10–22.
Li W, Woldu A, Kelly R, McCool J, Bruce R, Rasmussen H, et al. Measurement of drug agglomerates in powder blending simulation samples by near infrared chemical imaging. Int J Pharm. 2008;350(1–2):369–73.
Ma H, Anderson C. Optimisation of magnification levels for near infrared chemical imaging of blending of pharmaceutical powders. J Near Infrared Spectrosc. 2007;15(2):137.
Ma H, Anderson CA. Characterization of pharmaceutical powder blends by NIR chemical imaging. J Pharm Sci Us. 2008;97(8):3305–20.
Shi Z, Anderson CA. Application of Monte Carlo simulation-based photon migration for enhanced understanding of near-infrared (NIR) diffuse reflectance. Part II: photon radial diffusion in NIR chemical images. J Pharm Sci. 2010;99(10):4174–82.
Acknowledgments
The authors would like to thank Mr. Steve Lomuscio and Ms. Zeneida Go for sample preparations and Drs. Qingyan Hu and Raman Iyer for scientific discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, H., Choi, D.S., Zhang, YE. et al. Evaluation on the Drug–Polymer Mixing Status in Amorphous Solid Dispersions at the Early Stage Formulation and Process Development. J Pharm Innov 8, 163–174 (2013). https://doi.org/10.1007/s12247-013-9156-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-013-9156-z