Abstract
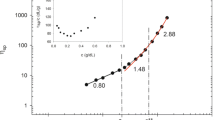
In this work, we present the evaluation of the thermotropic gelling ability and steady-state rheology of aqueous sodium alginate (NaAlg) solutions. NaAlg is been considered due to its gelling capabilities and to determine its suitability as a film-forming agent with the potential capacity for immobilization of very potent, solid, and hydrophobic drugs. The existence of a thermally induced gelation of 1.0 to 2.5 wt.% sodium alginate solutions between 13°C to 22°C was demonstrated through three independent rheological tests: the constant-stress temperature-ramp viscosity, dynamic viscoelastic moduli, and thixotropy tests. Oscillatory dynamic tests also showed the thermoreversible nature of the NaAlg gels, but a large hysteresis between the gel formation and the gel melting was observed. The steady-state viscosity was experimentally determined as a function of shear rate for NaAlg concentrations between 1.0 to 2.5 wt.% and for temperatures from 20°C (or above Tgel) to 90°C. A viscosity master curve was constructed by applying time–temperature superposition and a semi-empirical shift factor for concentration. A power-law fluid model (\( \eta = k{{\dot{\gamma }}^n} \)) was adjusted to the data from 20°C to 60°C and found to provide good agreement with fitting parameters k = 4.81 and n = −0.449 ± 0.003.







Similar content being viewed by others
References
Frey P. Film strips and pharmaceuticals. Pharmaceutical Manufacturer and Packing Sourcer. 2006. Winter:92–93
Vaczek D. New routes of drug delivery. In: Pharmaceutical and Medical Packaging News. 2007. p. 82–88.
McHugh DJ. A guide to the seaweed industry. FAO Fisheries Technical Paper, No 441. 2003, Rome.
Phillips GO, Williams PA, editors. Handbook of Hydrocolloids. Woodhead Publishing Limited/CRC Press LLC; 2000.
Braun DB, Rosen MR. Rheology modifiers handbook: practical use and application. New York: William Andrew Publishing; 2000.
Wang ZY et al. Sol-Gel transition of alginate solution by the addition of various divalent-cations—a rheological study. Biopolymers. 1994;34(6):737–46.
Liu XX et al. Rheology characterization of sol–gel transition in aqueous alginate solutions induced by calcium cations through in situ release. Polymer. 2003;44(2):407–12.
Lu L et al. Sol–gel transition in aqueous alginate solutions induced by cupric cations observed with viscoelasticity. Polym J. 2003;35(10):804–9.
de Kerchove AJ, Elimelech M. Formation of polysaccharide gel layers in the presence of Ca2+ and K+ ions: measurements and mechanisms. Biomacromolecules. 2007;8(1):113–21.
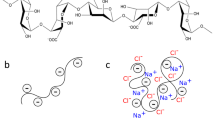
Grant GT et al. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 1973;32:195–8.
Braccini I, Perez S. Molecular basis of C2+-induced gelation in alginates and pectins: the egg-box model revisited. Biomacromolecules. 2001;2:1089–96.
Abbah SA et al. Osteogenic behavior of alginate encapsulated bone marrow stromal cells: an in vitro study. J Mater Sci Mater Med. 2008;19(5):2113–9.
Kost J, Goldbart R. Natural and modified polysaccharides. In: Domb AJ, Kost J, Wiseman DM, editors. Handbook of biodegradable polymers. Boca Raton: CRC Press; 1997.
Rao CS et al. Studies on improving the immobilized bead reusability and alkaline protease production by isolated immobilized Bacillus circulans (MTCC 6811) using overall evaluation criteria. Appl Biochem Biotechnol. 2008;150(1):65–83.
Wells LA, Sheardown H. Extended release of high pI proteins from alginate microspheres via a novel encapsulation technique. Eur J Pharm Biopharm. 2007;65(3):329–35.
Li TP et al. Optimization of covalent immobilization of pectinase on sodium alginate support. Biotechnol Lett. 2007;29(9):1413–6.
Yabur R, Bashan Y, Hernandez-Carmona G. Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J Appl Phycol. 2007;19(1):43–53.
Potumarthi R et al. Evaluation of various parameters of calcium-alginate immobilization method for enhanced alkaline protease production by Bacillus licheniformis NCIM-2042 using statistical methods. Bioresour Technol. 2008;99(6):1776–86.
Mladenovska K et al. 5-ASA loaded chitosan–Ca–alginate microparticles: preparation and physicochemical characterization. Int J Pharm. 2007;345:59–69.
Reis CP et al. Polyelectrolyte biomaterial interactions provide nanoparticulate carrier for oral insulin delivery. Drug Deliv. 2008;15(2):127–39.
Mitamura K et al. Fabrication and structure of alginate gel incorporating gold nanorods. J Phys Chem C. 2008;112(2):416–22.
Bhat SD, Aminabhavi TM. Zeolite K-LTL-loaded sodium alginate mixed matrix membranes for pervaporation dehydration of aqueous–organic mixtures. J Membr Sci. 2007;306(1–2):173–85.
Patil MB et al. Preparation and characterization of filled matrix membranes of sodium alginate incorporated with aluminum-containing mesoporous silica for pervaporation dehydration of alcohols. Sep Purif Technol. 2007;54(1):34–43.
Morch YA et al. Molecular engineering as an approach to design new functional properties of alginate. Biomacromolecules. 2007;8(9):2809–14.
Chan AW et al. Kinetic controlled synthesis of pH-responsive network alginate. Biomacromolecules. 2008;9:2536–45.
Aminabhavi TM, Agnihotri SA, Naidu BVK. Rheological properties and drug release characteristics of pH-responsive hydrogels. J Appl Polym Sci. 2004;94(5):2057–64.
Rao K et al. Controlled release of diclofenac sodium and ibuprofen through beads of sodium alginate and hydroxy ethyl cellulose blends. J Appl Polym Sci. 2006;102(6):5708–18.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114(1):1–14.
Voron’ko NG, Derkach SR, Izmailova VN. Rheological properties of gels of gelatin with sodium alginate. Russ J Appl Chem. 2002;75(5):790–4.
Xiao CB et al. Blend films from sodium alginate and gelatin solutions. J Macromol Sci Pure Appl Chem. 2001;38(3):317–28.
Soares JP et al. Thermal behavior of alginic acid and its sodium salt. Ecletica Quimica. 2004;29(2):57–63.
Sakugawa K et al. Simplified method for estimation of composition of alginates by FTIR. J Appl Polym Sci. 2004;93:1372–7.
Seok Y et al. Effect of flavor on the viscosity and gelling point of aqueous poloxamer solution. Arch Pharm Res. 2006;29:1171–8.
Ding P et al. The effect of temperature and composition on the interfacial tension and rheology of separated phases in gelatin/pullulan mixtures. Food Hydrocolloids. 2005;19(3):567–74.
Cho JY et al. Chitosan and glycerophosphate concentration dependence of solution behaviour and gel point using small amplitude oscillatory rheometry. Food Hydrocolloids. 2006;20(6):936–45.
Van den Bulcke AI et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1(1):31–8.
Schrieber R, Gareis H. Gelatine handbook: theory and industrial practice. Weinheim: Wiley; 2007.
Fernandez E et al. Rheological and thermal properties of agarose aqueous solutions and hydrogels. J Polym Sci B Polym Phys. 2008;46(3):322–8.
Morrison FA. Understanding rheology. New York: Oxford University Press; 2001.
Perry RH, Green DW, editors. Perry’s chemical engineer’s handbook, 7th ed. McGraw-Hill; 1997.
Kokini JL, Surmay K. Steady shear viscosity first normal stress difference and recoverable strain in carboxymethyl cellulose, sodium alginate and guar gum. Carbohydr Polym. 1994;23:27–33.
Larson RG. Structure and rheology of complex fluids. New York: Oxford University Press; 1999.
Morris ER et al. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydr Polym. 1981;1(1):5–21.
Seale R, Morris ER, Rees DA. Interactions of alginates with univalent cations. Carbohydr Res. 1982;110(1):101–12.
Lapasin R, Pricl S. Rheology of industrial polysaccharides: theory and applications. A Chapman and Hall Food Science Book. Maryland: Aspen Publishers, Inc; 1999.
Acknowledgment
The authors gratefully acknowledge the National Science Foundation (Grant 0540855) for support of this research conducted through the Engineering Research Center for Structured Organic Particulate Systems (C-SOPS) at the University of Puerto Rico at Mayagüez. We thank Sindia Rivera for providing help with the TGA and Dr. Carlos Rinaldi for the use of the rheometer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Florián-Algarín, V., Acevedo, A. Rheology and Thermotropic Gelation of Aqueous Sodium Alginate Solutions. J Pharm Innov 5, 37–44 (2010). https://doi.org/10.1007/s12247-010-9078-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-010-9078-y