Abstract
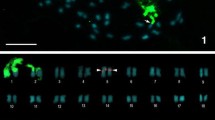
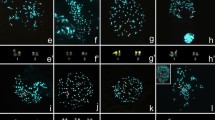
To assess the taxonomic utility of cytogenetic variation in the species-rich neotropical papilionoid legume genus Swartzia and to ascertain the importance of cytogenetic evolution in the diversification history of the genus, a variety of cytogenetic data—chromosome number, chromosome lengths, relative chromosome length, total chromatin length (TCL), CMA/DAPI and FISH—were collected for 19 taxa of Swartzia and for a single species of the related genus Ateleia. In the sampled species of Swartzia, chromosome counts yielded a diploid number of 2n=2x=26. However, both diploid and tetraploid (2n=4x=52) counts were obtained for S. leptopetala. The species of Swartzia presented small chromosomes (0.25μm to 1.41μm), with gradual length variation, furthermore, each of them had two sites of CMA+/DAPI- and two sites of 45S and 5S rDNA. Cytogenetic data for the morphologically anomalous species S. euxylophora convey its close relationship to other species of Swartzia. Ateleia ovata was found to differ from all of the sampled taxa of Swartzia in diploid chromosome number (2n =28). Taken together, these results constitute preliminary evidence for a strongly conserved karyotype pattern in Swartzia and in combination with previously published data suggest that karyological characters, while useful for characterizing the genus, are of limited taxonomic utility within Swartzia. We conclude that cytogenetic evolution involving changes in chromosome number has not figured prominently in the explosive diversification history of Swartzia.


Similar content being viewed by others
Literature cited
Biondo, E., S. T. S. Miotto & M. T. Schifino-Wittmann. 2005. Números cromossômicos e implicações sistemáticas em espécies da subfamília Caesalpinioideae (Leguminosae) ocorrentes na região Sul do Brasil. Revista Brasileira de Botânica 28: 797–808.
Bolkhoviskikh, Z., V. Grif, T. Matvejeva & H. V. Zakharyeva. 1969. Chromosome number of flowering plants. V. L. Komarov Botanical Institute. Academy of Sciences of the USSR, Moscou.
Bou Dagher-Kharrat, M., N. Abdel-Samad, B. Douaihy, M. Bourge, A. Fridlender, S. Siljak-Yakovlev & S. C. Brown. 2013. Nuclear DNA C-values for biodiversity screening: Case of the Lebanese flora. Plant Biosystems 147(4): 1228–1237.
Chao, Y. S., H. Y. Liu, Y. C. Chiang & W. L. Chiou. 2012. Polyploidy and speciation in Pteris (Pteridaceae). Journal of Botany: doi:10.1155/2012/817920.
Cusma-Velari, T. & L. Feoli-Chiapella. 2009. The so-called primitive genera of Genisteae (Fabaceae): systematic and phyletic considerations based on karyological data. Botanical Journal of the Linnean Society 160: 232–248.
Dahmer, N., M. F. Simon, M. T. Schifino-Wittmann, C. E. Hughes, S. T. S. Miotto & J. C. Giuliani. 2011. Chromosome numbers in the genus Mimosa L.: cytotaxonomic and evolutionary implications. Plant Systematics and Evolution 291: 211–220.
Doyle, J. J. 2012. Polyplody in Legumes. Pp. 147-180. In: P. S. Soltis & D. E. Soltis (eds.), Polyploidy and Genome Evolution. Springer Berlin Heidelberg, Germany.
Forni-Martins, E. R., M. Franchi-Tanibata & M. A. C. Lucena. 1994. Karyotypes of species of Sesbania Scop. (Fabaceae). Cytologia 59: 479–482.
Goldblatt, P. 1981. Cytology and the phylogeny of Leguminosae. Pp. 427-463. In: R. M. Polhill & P.H. Raven (eds.), Advances in legume systematics. Part 2. Kew: Royal Botanic Gardens, London, England.
Govindarajulu, R., C. E. Hughes & C. D. Bailey. 2011. Phylogenetic and population genetic analyses of diploid Leucaena (Leguminosae; Mimosoideae) reveal cryptic species diversity and patterns of divergent allopatric speciation. American Journal of Botany 98(12): 2049–2063.
Grant, V. 1981. Plant speciation. Columbia University Press, New York.
Guerra, M. & M. J. Souza. 2002. Como observar cromossomos: um guia de técnicas em citogenética vegetal, animal e humana. Fundação de Pesquisas Científicas de Riberião Preto, São Paulo.
———. 2009. Chromosomal variability and the origin of Citrus species. Pp. 51–68. In: C. L. Mahoney & D. A. Springer (eds.), Genetic diversity. Nova Science, New York.
Hipp, A. L., P. E. Rothrock & E. H. Roalson. 2009. The evolution of chromosome arrangements in Carex (Cyperaceae). The Botanical Review 75(1): 96–109.
Hughes, C. E., C. D. Bailey & A. S. Harris. 2002. Divergent and reticulate species relationships in Leucaena (Fabaceae) inferred from multiple data sources: insights into polyploid origins and nrDNA polymorphism. American Journal of Botany 89(7): 1057–1073.
Levin, D. 2002. The role of chromosomal change in plant evolution. Oxford University Press. Oxford.
Lewis, G. P. 1987. Legumes of Bahia. Kew: Royal Botanic Gardens, London.
Mangenot, S. & G. Mangenot. 1957. Nombres chromosomiques nouveaux chez diverses dicotyledones d'Afrique occidentale. Bulletin du Jardin botanique de l'État a Bruxelles 27(4): 639–654.
Mondin, M., J. A. Santos-Serejo & M. L. R. Aguiar-Perecin. 2007. Karyotype characterization of Crotalaria juncea (L.) by chromosome banding and physical mapping of 18S-5.8S-26S and 5S rRNA gene sites. Genetics and Molecular Biology 30(1): 65–72.
Moraes, A. P., I. J. Leitch & A. R. Leitch. 2012. Chromosome studies in Orchidaceae: karyotype divergence in Neotropical genera in subtribe Maxillariinae. Botanical Journal of the Linnean Society 170: 29–39.
Morales, M., A. F. Wulff, R. H. Fortunato & L. Poggio. 2014. Chromosome studies in southern species of Mimosa (Fabaceae, Mimosoideae) and their taxonomic and evolutionary inferences. Plant Systematics and Evolution 300(5): 803–817.
Naganowska, B., B. Wolko, E. Sliwinska & Z. Kaczmarek. 2003. Nuclear DNA Content Variation and Species Relationships in the Genus Lupinus (Fabaceae). Annals of Botany 92(3): 349–355.
Pedrosa, A., N. Sandal, J. Stougaard, D. Schweizer & A. Bachmair. 2002. Chromosomal map of the model legume Lotusjaponicus. Genetics Society of America 161(4): 1661–1672.
Pinto, R. B., B. M. Torke & V. F. Mansano. 2012. Updates to the taxonomy of Swartzia(Leguminosae) in extra-Amazonian Brazil, with description of five new species and a regional key to the genus. Brittonia 64(2): 119–138.
Santos, E. C. X. R., R. Carvalho, E. M. Almeida & L. P. Felix. 2012. Chromosome number variation and evolution in Neotropical Leguminosae (Mimosoideae) from northeastern Brazil. Genetics and Molecular Research 11: 2451–2475.
Seijo, J. G. & A. Fernández. 2003. Karyotype analysis and chromosome evolution in South American species of Lathyrus(Leguminosae). American Journal of Botany 90(7): 980–987.
Soltis, D. E., P. S. Soltis & J. A. Tate. 2003. Advances in the study of polyploidy since plant speciation. New Phytology 161: 173–191.
Souza, L. G. R., O. Crosa & M. Guerra. 2012. Karyological circumscription of Ipheion Rafinesque (Gilliesioideae, Alliaceae). Plant Systematics and Evolution 287(3): 119–127.
Stebbins, G. L. 1971. Chromosomal evolution in higher plants. Edward Arnold Ltd., London.
Torke, B. M. 2006. Systematics and evolutionary diversification in the species-rich neotropical tree genus Swartzia (Leguminosae - Papilionoideae). PhD diss. Washington University, St. Louis, Missouri, USA.
——— & B. Schaal. 2008. Molecular phylogenetics of the swartzioid clade (Leguminosae-Papilionoideae) revisited and a phylogenetic hypothesis for the species-rich Neotropical genus Swartzia. American Journal of Botany 95: 215–228.
——— & V. F. Mansano. 2009. A phylogenetically based sectional classification of Swartzia (Leguminosae-Papilionoideae). Taxon 58: 913–924.
Vickery, R. K., Jr. 1995. Speciation by aneuploidy and polyploidy in Mimulus (Scrophulariaceae). Great Basin Naturalist 55(2): 174–176.
Wanzenböck, E. M., C. Schöfer, D. Schweiser & A. Bachmair. 1997. Ribosomal transcription units integrated via T-DNA transformation associate with the nucleolus and do not require upstream repeat sequences for activity in Arabidopsis thaliana. Plant Journal 11(5): 1007–1016.
Wood, T. E., N. Takebayashic, M. S. Barker, I. Mayrosee, P. B. Greenspoond & L. H. Rieseberg. 2009. The frequency of polyploid speciation in vascular plants. Proceedings of the National Academy of Sciences of the United States of America 106: 13875–13879.
Acknowledgments
We gratefully acknowledge the financial support of the Post-Graduate Program of the Universidade Estadual de Campinas (PPG-UNICAMP), the Fundação de Apoio ao Ensino, Pesquisa e Extensão (FAEPEX-UNICAMP), and fellowships sponsored by grants to ERFM and VFM (process numbers 306142/2011-2 and 309987/2012-1, respectively) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). RBP is grateful to the Programa de Capacitação em Taxonomia (PROTAX), for providing his Master’s scholarship (process number 562280/2010-3). Herbarium and field studies were also supported by a grant from the National Science Foundation (NSF DEB-0918498, PIs BMT and VFM) and from the National Geographic Society Committee for Research and Exploration (NGS CRE grant no. 8770-10, PI BMT). We thank Dr. Itayguara Costa (Universidade Federal do Ceará - UFC), Msc. Jorge Tamashiro (UNICAMP), Dr. Adriana Lobão (Universidade Federal Fluminense - UFF), and several other colleagues for providing seeds for this work, Dr. Alberto Vicentini (Instituto Nacional de Pesquisas da Amazônia - INPA) for assistance during fieldtrips near Manaus, and Dr. Ana Maria Goulart de Azevedo Tozzi (UNICAMP) for sharing her knowledge on Fabaceae and Swartzia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinto, R.B., de Freitas Mansano, V., Torke, B.M. et al. Evidence for a conserved karyotype in Swartzia (Fabaceae, Papilionoideae): Implications for the taxonomy and evolutionary diversification of a species-rich neotropical tree genus. Brittonia 68, 93–101 (2016). https://doi.org/10.1007/s12228-015-9395-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12228-015-9395-z