Abstract
The surveillance study of rotavirus gastroenteritis at the University Teaching Hospital Trenčín area, Slovakia, during 2006–2011 confirmed that the genotype profile of circulating rotaviruses was not stable. While G1P[8] dominating genotype dropped from 75 to 7.3 % in the period 2009–2011, genotype G2P[4] that was not detected in 2009 raised to 45.1 % in 2011. Vaccination coverage rose from 4.4 to 22.1 % in the period 2008–2011. Among the community and hospital cases, we observed that the average age of patients with nosocomial infections was significantly less (10.6 months) than in the cases of community rotavirus gastroenteritis (RVGE) cases. Compared to the nosocomial infection cases, the duration of the disease and the duration of hospitalization among the community cases were significantly longer by 0.22 and 3.63 days, respectively, during 2006–2011. Though the vaccination coverage was found to correlate with changes in the type of the circulating rotaviruses, the natural circulation in rotavirus genotypes may not be excluded as important factor contributing to the emergence of G2P[4] strain during the survey period.
Similar content being viewed by others
Introduction
Rotaviruses are the most common etiological agent causing severe gastroenteritis in children less than 5 years of age. The infection results in a threefold higher rate of hospitalization than gastroenteritis caused by other agents (Ehlken et al. 2002).
In the European region, there is a dynamic seasonal pattern of rotavirus infections, spreading from October to May with accumulation from February to April. Faecal-oral transmission is the dominant mode of transmission for spread of the infection. Rotavirus enteritis present clinically as light-to-severe diarrhoea with vomiting and high fever. Hospitalization is required in most cases, and a fatal outcome is not uncommon (Beran et al. 2008). There is only partial immunity after the first infection, and reinfections can occur, but usually with milder symptoms.
Rotaviruses are divided into seven serological groups A–G according to the antigens located in the specific central capsid proteins of the virus particle. The most important group for humans is group A with 31 serotypes of proteins split by proteinases (P type) and 23 serotypes of the glycoproteins (G type). The P and G types can produce 132 antigenic combinations (Beran et al. 2008; Desai and Vázquez 2010). From the viewpoint of pathogenic potential, the most important combinations for humans are G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8], and these five combinations produce more than 90 % of all cases (Santos and Hoshino 2005). Among the circulating rotaviruses, there is a marked fluctuation in the genotype profile, both geographically and over time (Iturriza-Gómara et al. 2011).
Since the introduction of the rotavirus vaccination programme, it is important to monitor the dynamic nature of the disease and to monitor the development of new genetic recombinant types of the virus that may occur as a result of this programme. (Iturriza-Gómara et al. 2011; Pazdiora and Svecova 2006).
Here we present information obtained in a prospective study focused on cases of rotavirus gastroenteritis (RVGE) in the University Teaching Hospital Trenčín (UTHT) area in Western Slovakia which has 236,902 inhabitants. We present profiles of the types of rotavirus associated with both nosocomial and community RVGE in a vaccinated population and their associated clinical and epidemiological characteristics.
Material and methods
Subjects
Children, ages 0–5, who were admitted to the Children’s Clinic, UTHT, during period 2006–2011 with symptoms of gastroenteritis which was confirmed by laboratory testing as having a rotavirus aetiology were enrolled in the study. A total of 10,355 children in this age range were living in the UTHT area during this period.
Nosocomial RVGE was defined
-
when the child was admitted with a diagnosis other than gastroenteritis
-
when the first symptoms of RVGE appeared not earlier than 24 h after admission
-
when the family reported no signs of diarrhoeal diseases
-
when the child was re-hospitalized at the Children’s Clinic within 3 days (incubation period for RVGE) with symptoms of gastroenteritis after the first admission
-
when RVGE was confirmed by ELISA
Laboratory tests
Stool samples from hospitalized children were tested for the presence of rotavirus by the strip ELISA (Coris BioConcept, Belgium). Positive samples taken during the period 2009–2011 were further tested using real-time PCR (RT-PCR) with primers specific for G1, G2, G3, G4, G9, P4, P6 and P8 (van der Heide et al. 2005; Gentsch et al. 1992; Gómara et al. 2001; Das Das et al. 1994).
Data analysis
For epidemiological and clinical analysis, the information from children with RVGE was collected by questionnaire prepared using the Epi Info programme (CDC Atlanta, USA). The information included age, season, and the number in the ICU, the period of the infusion therapy, and episodes of diarrhoea, vomiting and fever. Differences between nosocomial and community RVGE were evaluated using the t test (P < 0.05). Vaccination rates were calculated as the number of doses applied in the study area during the period 2008–2011. The two vaccines that were used were Rotarix (GlaxoSmithKline) and RotaTeq (Merck Sharp & Dohme).
Results
Of the 10,355 children, age 0–5, living in the study area during the period 2006–2011, 6196 were hospitalized at the Children’s Clinic UTHT. Gastroenteritis was diagnosed in 1573 (25.38 %), and rotavirus aetiology was confirmed in 461 children, representing 29.3 % of all gastroenteritis and 7.4 % of hospitalized children. There was no case of RVGE in vaccinated children during the study period.
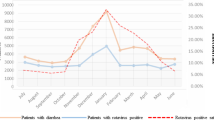
Community RVGE was diagnosed in 354 cases (76.8 %) while nosocomial RVGE was diagnosed in 107 cases (23.2 %) (Fig. 1). The average annual incidence of nosocomial RVGE was 1.03 per 100 children, age 0–5. Thirty-eight appeared at home after the patient was discharged from the hospital and was readmitted to the hospital. These cases represented 8.2 % of all RVGE and 35.5 % of all nosocomial RVGE.
The zero–five-year age group was 85/10,000 (45–126/10,000). The average age of the children was 24.62 months (range 0–60 months) while the average age of children with nosocomial RVGE was 16.51 months (range 0–53 months). The average age of children with community RVGE was 27.15 months (range 0–60 months). The majority of children, 258 (56 % from 461), with RVGE contracted the infection before reaching 24 months of age and 348 (75.5 %) before reaching the 36 months. From the total number of 107 nosocomial RVGE cases, 85 (79.4 %) were 2 years or younger and 97 (90.7 %) were 3 years or younger. From the total number of 354 community RVGE cases, 173 (48.8 %) were 2 years or younger and 251 (70.9 %) were 3 years or younger (Fig. 1).
We have confirmed 246 rotavirus positive samples (107, 47 resp. 92) from which 186 (68, 36 resp. 82) were further tested using RT-PCR during the period 2009–2011. This represents 75.6 % (63.6, 76.6 resp. 89.1 %) from all samples. There was a significant decrease in the presence of genotype G1P[8] from 75 % in 2009 to 7.3 % in 2011. In contrast, genotype G2P[4] significantly increased from 0 to 45.1 % during the same period. The other genotypes revealed no significant fluctuations (Fig. 2). The seasonal time course of the RVGE displayed two peaks. The main one is in spring (February–March) with 65.7 % of cases, and the second one is in the fall (October–December) with 14.8 % of cases.
Three characteristics were found to exhibit significant differences between nosocomial and community RVGE. In the group of nosocomial RVGE, the average age of the patients was by 10.6 months lower, the average duration of the disease was 0.22 day longer and the duration of hospitalization was 3.63 days longer in comparison with the community RVGE. No significant differences between the community and nosocomial RVGE groups were found in regard to stay in the ICU, days of infusion therapy, number of diarrhoeal episodes or vomiting and duration of the fever. (Table 1).
Discussion
To our knowledge, this is the first prospective study of the RVGE in the Slovak Republic analysed by the ELISA and RT-PCR. Three dominating groups, covered three serotypes and two genotypes, were found, G1P[8], G4P[8] and G2P[4] (Figs. 2 and 3). There are four prevailing G-group types (G1, G2, G3, G4) that, in combination with P8 a P4 serotypes, are responsible for 88 % of RVGE worldwide. In Europe, the genotype G1P[8] has been reported to be responsible for 71.6 % of RVGE (Santos and Hoshino 2005). Geographic and seasonal G1 and G4 variability has been observed in most of the EU countries. Genotype G1 was dominant in the Czech Republic in the 1999–2002 (Pazdiora and Svecova 2006). Genotype G1P[8] was reported in Bulgaria (39.5 %), Russia (47 %), Slovenia (75 %) and Ukraine (32 %) during the 2007–2008 season. G2P[4] was reported only from Russia (4.0 %) and Bulgaria (25.8 %) (Van Damme et al. 2007b).
Rotavirus genotyping started in Slovakia in 2009 when G1P[8] was detected in 75 %. No G2P[4] was detected at that time. The next year, however, the G1P[8] declined steadily reaching 7.3 % in 2011. In contrast, G2P[4] increased to 45.1 % during this period. Austria reported 73 % of G2P[4] in 2011, and a similar pattern was reported for this period in Belgium. It is interesting to see the parallel between vaccination coverage and the spread of rotavirus types. In Austria when G2P[4] was responsible for 73 % of RVGE, the vaccination coverage reached 80 %. In Slovakia, with vaccination coverage at 22.1 % in 2011, the G2P[4] was responsible for 45.1 % of RVGE. Thus, it appears that rotavirus vaccine suppresses the spread of the G1P[8] type, leaving more ecospace for the G2P[4] type. This genotype shows also a higher prevalence among older children. Lower cross-protective immunity stimulated by the natural infection caused by the circulating human genotypes is probably responsible for this phenomenon (Iturriza-Gómara et al. 2011). The emergence of G2P[4] has been reported in vaccinated population (Brazil—Rotarix, Nicaragua—RotaTeq), but also in non-vaccinated populations in Latin America. In Australia, the dominance of G2P[4] was associated with a large outbreaks of severe gastroenteritis in Northern Territory in 2009 (Kirkwood et al. 2009).
Previous studies point to the protective effect of vaccination against the RVGE in children, including non-vaccinated ones, provided that the coverage is sufficiently high in the specific region (CDC MMWR, 2009; Lambert et al. 2009; O’Ryan et al. 2011; Patel et al. 2011; Pazdiora 2010). In Slovakia, the vaccination programme was started in 2008, and coverage in the study area doubled yearly and is currently at 22.1 %. Throughout the Slovak Republic, the vaccination coverage increased from 4 to 13.8 % in 2013. Yearly hospitalization for RVGE in the study area reached 0.85/100 children, age 0–5 (range 0.45–1.26). Similar hospitalization rates were reported in the UK, Germany and Spain. Project REVEAL indicated that in 7 EU countries, the range of vaccinations was 0.29–099/100,000. (Van Damme et al. 2007a; Gil et al. 2004; Poppe et al. 2002; Ryan et al. 1996).
Nosocomial RVGE (Gleizes et al. 2006) rates in six European countries ranged between 0.3 and 27.7 % of all hospitalizations. In our study, RVGE represented 7.4 % of all hospitalizations during the period 2006–2011. The incidence of nosocomial RVGE ranged between 0.16 and 0.63 cases/100 children, and the average yearly incidence of nosocomial RVGE was 1.03/100 children, indicating no striking differences in comparison with other European studies.
The average age of the children with the nosocomial RVGE was 10.64 months lower than in children with community-type RVGE. There are several factors that have an impact on the prevention of nosocomial infections. One study showed that by strict application of a disinfection programme, the number of nosocomial RVGE cases decreased from 5.9 to 2.2 episodes (Zerr et al. 2005). Some of the factors that may contribute to a decreased effectiveness of hygienic and epidemiological preventive measures include rotavirus resistance to biocides and environmental factors and asymptomatic infections in the elderly that often go unrecognized as a source of infection.
Hospitalizations due to the RVGE in our study did not show any significant differences from those (1.7–5.9 days) found in other European studies. Similarly, the re-hospitalization rate in these studies due to the occurrence of nosocomial RVGE after the patient was discharged was similar (2–13 %). (Gleizes et al. 2006).
The present study shows the variability of rotavirus genotype profile in children during the period 2009–2011 in UTHT area. The dominating genotype G1P[8] decreased from 75 drop to 7.3 %, while genotype G2P[4] increased from 0 to 45.1 %. These changes reflected increased vaccination coverage; however, to determine a causal association would be premature due to the presence of other factors including low vaccination coverage (22.1 %). It will be important to observe whether G2P[4] continues to remain a common genotype. If not, then its emergence during the survey period will be more likely due to the natural circulation in rotavirus genotypes, than to the vaccine pressure.
Our study points out to the potential of RVGE surveillance to produce data that is important for vaccine development and for developing preventive and intervention measures that are critical for effective control of both community and nosocomial infections.
References
Beran J, Havlík J et al (2008) Lexikon očkování. 1. vyd. Praha, Maxdorf, p 352
Centers for Disease Control and Prevention (CDC) Reduction in rotavirus after vaccine introduction—United States, 2000–2009. MMWR Morb. Mortal. Wkly. Rep., 2009, Vol. 41, No 58, pp. 1146–1149
Das BK, Gentsch JR, Cicirello HG et al (1994) Characterization of rotavirus strains from newborns in New Delhi. India J Clin Microbiol 32(7):1820–1822
Desai SN, Vázquez M (2010) Update on rotavirus trends and the importance of surveillance. Pediatr Infect Dis J 12(29):1130–1132
Ehlken B, Laubereau B, Karmaus W, Petersen G, Rohwedder A, Forster J (2002) Prospective population-based study on rotavirus disease in Germany. Acta Paediatr 7(91):769–775
Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, Das BK, Bhan MK (1992) Identification of Group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 30(6):1365–1373
Gil A, Carrasco P, Jiménez R, San-Martin M, Oyagüez I, Gonzalez A (2004) Burden of hospitalizations attributable to rotavirus infection in children in Spain, period 1999–2000. Vaccine 2(22):2221–2225
Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, Giaguinto C, Grimprel E (2006) Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J 1(25):12–21
Gómara MI, Cubitt D, Desselberger U, Gray J (2001) Amino acid substitution within the VP7 protein of G2 rotavirus strains associated with failure to serotype. J Clin Microbiol 39(10):3796–3798
Iturriza-Gómara M, Dallman T, Bányai K, Böttiger B, Buesa J et al (2011) Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol Infect 139(6):895–909
Kirkwood CD, Boniface K, Bishop RF, Barnes GL, Australian Rotavirus Surveillance Group (2009) Australian Rotavirus Surveillance Program annual report, 2008/2009. Commun Dis Intell Q Rep 33(4):382–388
Lambert SB, Faux CE, Hall L, Birrell FA, Peterson KV, Selvey CE, Sloots TP, Nissen MD, Grimwood K (2009) Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust 3:157–160
O’Ryan M, Lucero Y, Linhares AC (2011) Rotarix®: vaccine performance 6 years postlicensure. Expert Rev Vaccines 12(10):1645–1659
Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD (2011) Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 30(Suppl. 1):1–5
Pazdiora P (2010) Vakcinace proti rotavíusovým infekcím. Vakcinologie 4:159–166
Pazdiora P, Svecova M (2006) G-serotypes of group A rotaviruses in Pilsen region (Czechia). Folia Microbiol 2(51):133–135
Poppe M, Ehlken B, Rohwedder A et al (2002) Morbidity and hospital admissions due to rotavirus infection in Germany. Monatsschr Kinderheilkd 150:491–496
Ryan MJ, Ramsay M, Brown D, Gay NJ, Farrington CP, Wall PG (1996) Hospital admissions attributable to rotavirus infection in England and Wales. J Infect Dis 1(174):12–18
Santos N, Hoshino Y (2005) Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 1(15):29–56
Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M et al (2007a) Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis 195(1):4–16
Van Damme P, Giaquinto C, Maxwell M, Todd P, der Wielen V (2007b) Distribution of rotavirus genotypes in Europe, 2004–2005. J Infect Dis 1(195):17–25
van der Heide R, Koopmans MP, Shekary N, Houwers DJ, van Duynhoven YT, van der Poel WH (2005) Molecular characterizations of human and animal group A rotaviruses in the Netherlands. J Clin Microbiol 43(2):669–675
Zerr DM, Allpress AL, Heath J, Bornemann R, Bennett E (2005) Decreasing hospital-associated rotavirus infection: a multidisciplinary hand hygiene campaign in a children’s hospital. Pediatr Infect Dis J 5(24):397–403
Acknowledgments
We thank prof. Tom Cook for the critical reading of the manuscript and English editoring assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Štefkovičová, M., Litvová, S., Šimurka, P. et al. Rotavirus type profile in nosocomial and community infections in Western Slovakia. Folia Microbiol 60, 177–181 (2015). https://doi.org/10.1007/s12223-014-0358-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-014-0358-7