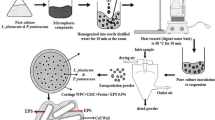
Abstract
The purpose of this study was to improve the survival of Bifidobacterium animalis subsp. lactis 10140 during freeze-drying process by microencapsulation, using a special pediatric prebiotics mixture (galactooligosaccharides and fructooligosaccharides). Probiotic microorganisms were encapsulated with a coat combination of prebiotics–calcium-alginate prior to freeze-drying. Both encapsulated and free cells were then freeze-dried in their optimized combinations of skim milk and prebiotics. Response surface methodology (RSM) was used to produce a coating combination as well as drying medium with the highest cell viability during freeze-drying. The optimum encapsulation composition was found to be 2.1 % Na-alginate, 2.9 % prebiotic, and 21.7 % glycerol. Maximum survival predicted by the model was 81.2 %. No significant (p > 0.05) difference between the predicted and experimental values verified the adequacy of final reduced models. The protection ability of encapsulation was then examined over 120 days of storage at 4 and 25 °C and exposure to a sequential model of infantile GIT conditions including both gastric conditions (pH 3.0 and 4.0, 90 min, 37 °C) and intestinal conditions (pH 7.5, 5 h, 37 °C). Significantly improved cell viability showed that microencapsulation of B. lactis 10140 with the prebiotics was successful in producing a stable symbiotic powdery nutraceutical.






Similar content being viewed by others
References
Abe F, Miyauchi H, Uchijima A, Yaeshima K, Iwatsuki K (2009) Effect of storage temperature and water activity on survival of bifidobacteria in powder form. Inter J Dairy Tech 62:234–239
Adamson AW (1982) Physical chemistry of surfaces. Wiley, New York
Annan N, Borza A, Hansen L (2008) Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703 T during exposure to simulated gastro-intestinal conditions. Food Res Int 41(2):184–193
Arslanoglu S, Moro G, Boehm G (2007) Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr 137(11):2420–2425
Arslanoglu S, Moro G, Schmitt J, Tandoi L, Rizzardi S, Boehm G (2008) Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr 6:1091–1097
Bielecka M, Biedrzycka E, Majkowska A (2002) Selection of probiotics and prebiotics for synbiotics and confirmation of their in vivo effectiveness. Food Res Int 35(2–3):125–131
Broadbent J, Lin C (1999) Effect of heat shock or cold shock treatment on the resistance of Lactococcus lactis to freezing and lyophilization. Cryobiol 39(1):88–102
Carvalho A, Silva J, Ho P, Teixeira P, Malcata F, Gibbs P (2003) Effect of various growth media upon survival during storage of freeze-dried Enterococcus faecalis and Enterococcus durans. J Appl Microbiol 94(6):947–952
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2004) Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int Dairy J 14(10):835–847
Chandramouli V, Kailasapathy K, Peiris P, Jones M (2004) An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Meth 56(1):27–35
Charteris W, Kelly P, Morelli L, Collins J (1998) Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 84(5):759–768
Chen K, Chen M, Liu J, Lin C, Chiu H (2005) Optimization of incorporated prebiotics as coating materials for probiotic microencapsulation. J Food Sci 70(5):M260–M266
Coppa G, Bruni S, Morelli L, Soldi S, Gabrielli O (2004) The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol 38:S80–S83
Corcoran B, Stanton C, Fitzgerald G, Ross R (2005) Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl Environ Microbiol 71(6):3060–3067
Dave R, Shah N (1997) Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int Dairy J 7(1):31–41
Ding W, Shah N (2009) Effect of various encapsulating materials on the stability of probiotic bacteria. J Food Sci 74(2):M100–M107
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:33–50
FAO/WHO (2001) Report of a joint FAO/WHO expert consultation on “Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria”
Gilliland S, Speck M (1977) Instability of Lactobacillus acidophilus in yogurt. J Dairy Sci 60(9):1394–1398
Gueimonde M, Laitinen K, Salminen S, Isolauri E (2007) Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatology 92(1):64–66
Harmsen H, Wildeboer-Veloo A, Raangs G, Wagendorp A, Klijn N, Bindels J, Welling G (2000) Analysis of intestinal biota development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 30(1):61–67
Huang L, Lu Z, Yuan Y, Lü F, Bie X (2006) Optimization of a protective medium for enhancing the viability of freeze-dried Lactobacillus delbrueckii subsp. bulgaricus based on response surface methodology. J Ind Microbiol Biot 33(1):55–61
Joglekar AM, May AT (1987) Product excellence through design of experiments. Cereal Foods World 32(12):857–868
Kailasapathy K (2002) Microencapsulation of probiotic bacteria: technology and potential applications. Curr Iss Intest Microbiol 3(2):39–48
Kearney L, Upton M, Mc Loughlin A (1990) Enhancing the viability of Lactobacillus plantarum inoculum by immobilizing the cells in calcium-alginate beads incorporating cryoprotectants. Appl Environ Microbiol 56(10):3112–3116
Kebary K, Hussein S, Badawi R (1998) Improving viability of bifidobacteria and their effect on frozen ice milk. Egypt J Dairy Sci 26:319–338
Kim KI, Yoon YH (1995) A study on the preparation of direct vat lactic acid bacterial starter. Korean J Dairy Sci 17(2):129–134
Kim H, Kamara B, Good I, Enders G (1988) Method for the preparation of stabile microencapsulated lactic acid bacteria. J Ind Microbiol Biot 3(4):253–257
Kotikalapudi B (2009) Characterization and encapsulation of probiotic bacteria using a pea-protein alginate matrix. Master thesis, University of Saskatchewan. Saskatoon, Saskatchewan, Canada
Krasaekoopt W, Bhandari B, Deeth H (2003) Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J 13(1):3–13
Krückeberg St, Weißbrodt J, Kunz B. Mikroverkapselung bioaktiver Substanzen für die Nutzung in der Lebensmittelverarbeitung (Poster). 20 Jahrestagung der Biotechnologen, Juni 2002, Wiesbaden, Germany
Lee J, Ye L, Landen W (2000) Optimization of an extraction procedure for the quantification of vitamin E in tomato and broccoli using response surface methodology. J Food Compost Anal 13(1):45–57
Lian W, Hsiao H, Chou C (2003) Viability of microencapsulated bifidobacteria in simulated gastric juice and bile solution. Int J Food Microbiol 86(3):293–301
Martin R, Jimenez E, Heilig H, Fernandez L, Marin M, Zoetendal E, Rodriguez J (2008) Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-DGGE and qRTi-PCR. Appl Environ Microbiol 75:965–969
Mirhosseini H, Tan C, Hamid N, Yusof S (2008) Optimization of the contents of Arabic gum, xanthan gum and orange oil affecting turbidity, average particle size, polydispersity index and density in orange beverage emulsion. Food Hydrocolloids 22(7):1212–1223
Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, Boehm G (2002) Dosage-related bifidogenic effects of galacto-and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr 34(3):291–295
Mortazavian A, Razavi S, Ehsani M, Sohrabvandi S (2007) Principles and methods of microencapsulation of probiotic microorganisms. Iran J Biotechnol (IJB) 5(1):1–18
Mosilhey S (2003) Influence of different capsule materials on the physiological properties of microencapsulated Lactobacillus acidophilus. Ph.D. thesis, University of Bonn
Muller JA, Ross RP, Fitzgerald GF, Stanton C (2009) Prebiotics and probiotics science and technology, manufacture of probiotic bacteria. Charalampopoulos D and Rastall RA (eds) Springer, pp. 725-59
Orrhage K, Nord C (1999) Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatrica 88:47–57
Otero M, Espeche M, Nader-Macias M (2007) Optimization of the freeze-drying media and survival throughout storage of freeze-dried Lactobacillus gasseri and Lactobacillus delbrueckii subsp. delbrueckii for veterinarian probiotic applications. Process Biochem 42(10):1406–1411
Reuter G (1990) Bifidobacteria cultures as components of yoghurt-like products. Bifidobacteria Microflora 9(2):107–118
Roberfroid MB (2001) Prebiotics: preferential substrates for specific germs? Am J Clin Nutr 73(2):406–409
Saarela M, Lähteenmäki L, Crittenden R, Salminen S, Mattila-Sandholm T (2002) Gut bacteria and health foods—the European perspective. Int J Food Microbiol 78(1–2):99–117
Saarela M, Virkajärvi I, Alakomi H, Mattila Sandholm T, Vaari A, Suomalainen T, Mättö J (2005) Influence of fermentation time, cryoprotectant and neutralization of cell concentrate on freeze-drying survival, storage stability, and acid and bile exposure of Bifidobacterium animalis ssp. lactis cells produced without milk based ingredients. J Appl Microbiol 99(6):1330–1339
Schillinger U (1999) Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int J Food Microbiol 47(1–2):79–87
Schwab C, Vogel R, Gänzle M (2007) Influence of oligosaccharides on the viability and membrane properties of Lactobacillus reuteri TMW1. 106 during freeze-drying. Cryobiol 55(2):108–114
Shah N, Lankaputhra W (1997) Improving viability of Lactobacillus acidophilus and Bifidobacterium spp. in yogurt. Int Dairy J 7(5):349–356
Shah N, Ravula R (2000) Microencapsulation of probiotic bacteria and their survival in frozen fermented dairy desserts. Aust J Dairy Technol 55(3):139–144
Shamekhi F, Shuhaimi M, Ariff A, Manap YA (2011) Optimization of a cryoprotective medium for infant formula probiotic applications using response surface methodology. Ann Microbiol Pages 1–11. doi:10.1007/s13213-011-0328-0
Sheu T, Marshall R (1993) Microentrapment of lactobacilli in calcium alginate gels. J Food Sci 58(3):557–561
Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K (2000) Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol 62(1–2):47–55
Sun W, Griffiths M (2000) Survival of bifidobacteria in yogurt and simulated gastric juice following immobilization in gellan–xanthan beads. Int J Food Microbiol 61(1):17–25
Sung HH (1997) Enhancing survival of lactic acid bacteria in ice cream by natural encapsulation. Diss Abstr Int 13(9):5407
Thompson DB (2000) Strategies for the manufacture of resistant starch. Trends Food Sci Technol 11:245–253
Valerio F, De Bellis P, Lonigro S, Morelli L, Visconti A, Lavermicocca P (2006) In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl Environ Microbiol 72(4):3042
Wang YC, Yu RC, Chou CC (2004) Viability of lactic acid bacteria and bifidobacteria in fermented soymilk after drying, subsequent rehydration and storage. Int J Food Microbiol 93:209–217
Weng W, Liu W, Lin W (2001) Studies on the optimum models of the dairy product Kou Woan Lao using response surface methodology. Asian Aust J Anim Sci 14(10):1470–1476
Zhao G, Zhang G (2005) Effect of protective agents, freezing temperature, rehydration media on viability of malolactic bacteria subjected to freeze drying. J Appl Microbiol 99(2):333–338
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamekhi, F., Shuhaimi, M., Ariff, A. et al. Cell viability of microencapsulated Bifidobacterium animalis subsp. lactis under freeze-drying, storage and gastrointestinal tract simulation conditions. Folia Microbiol 58, 91–101 (2013). https://doi.org/10.1007/s12223-012-0183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-012-0183-9