Abstract
The photocatalytic reduction of CO2 is a promising strategy to generate chemical fuels. However, this reaction usually suffers from low photoactivity because of insufficient light absorption and rapid charge recombination. Defect engineering has become an effective approach to improve the photocatalytic activity. Herein, ultra-thin (~ 4.1 nm) carbon-doped Bi2WO6 nanosheets were prepared via hydrothermal treatment followed by calcination. The ultra-thin nanosheet structure of the catalyst not only provides more active sites but also shortens the diffusion distance of charge carriers, thereby suppressing charge recombination. Moreover, carbon doping could successfully extend the light absorption range of the catalyst and remarkably promote charge separation, thus inhibiting recombination. As a result, the as-prepared Bi2WO6 photocatalyst with ultra-thin nanosheet structure and carbon doping exhibits enhanced photocatalytic CO2 reduction performance, which is twice that of pristine ultra-thin Bi2WO6 nanosheet. This study highlights the importance of defect engineering in photocatalytic energy conversion and provides new insights for fabricating efficient photocatalysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The massive consumption of fossil fuels over the last several decades has led to the energy crisis and global warming [1, 2]. The photocatalytic conversion of CO2 into chemical fuels is widely believed to be a very promising approach to solve energy and environmental problems [3,4,5,6,7,8]. Although several photocatalysts, including CdS [9], TiO2 [10], ZnIn2S4 [11], and polymeric carbon nitride [12, 13], have been explored, the serious recombination of photogenerated charge carriers in these materials limits their photocatalytic efficiency [14, 15].
Bismuth tungstate (Bi2WO6), the simplest among the Aurivillius oxides, has attracted great attention on account of its layered structure, non-toxicity, and chemical stability [16, 17]. Despite these advantages, however, pristine Bi2WO6 exhibits only moderate photocatalytic CO2 activity because of extensive charge recombination. Therefore, various strategies, including morphology design [18], heteroatom doping [19, 20], heterojunction construction [21, 22], and coupling with cocatalysts [23], have been developed to enhance the photocatalytic CO2 reduction activity. Studies have confirmed that heteroatom doping is an effective approach to enhance the photocatalytic performance of Bi2WO6.
Herein, we propose a facile hydrothermal and calcination method to prepare ultra-thin carbon-doped Bi2WO6 nanosheets (Bi2WO6s) with improved photocatalytic CO2 reduction activity. The thickness of the C-doped Bi2WO6 nanosheets (C-Bi2WO6s) is within ~ 4.1 nm. Carbon doping not only successfully extended the light absorption range of the nanosheet but also greatly promoted charge separation, thereby inhibited recombination. The ultra-thin Bi2WO6 nanosheet exhibited relatively high CO2 photoreduction performance as a result of its increased active sites and excellent charge separation.
Experimental
Preparation of C-Bi2WO6s
C-Bi2WO6s were prepared by a facile hydrothermal and calcination method. In brief, 0.5 mmol of Na2WO4·2H2O and 0.025 g of hexadecyltrimethylammonium bromide (CTAB) were added to 40 mL of deionized water and mixed to obtain a solution. Then, 1 mmol of Bi(NO3)3·5H2O was added to the above solution, and the suspension was magnetically stirred for 60 min. The suspension was poured into a 50 mL Teflon-lined autoclave and hydrothermally treated at 120 °C for 24 h. The reactor was cooled to room temperature naturally, and the obtained sample was collected, washed three times with deionized water, and then dried at 60 °C in a vacuum oven for 12 h. The dry powder was placed in a tube furnace and calcined in a nitrogen gas atmosphere at 350 °C for 1 h with a heating rate of 3 °C/min. Finally, the obtained powder was denoted as C-Bi2WO6s. Carbon doping was realized by the carbonization of long carbon links in the residual CTAB.
Results and Discussion
Structure and Morphology
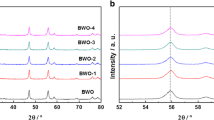
The X-ray diffraction (XRD) patterns of bulk Bi2WO6, pristine Bi2WO6s, and C-Bi2WO6s are shown in Fig. 1. The diffraction peaks of the samples could be indexed to those of orthorhombic Bi2WO6 (JCPDS Card No. 73–2020) [16]. No diffraction peaks assigned to carbon were observed in the XRD patterns, thus suggesting the high dispersibility of the element [24]. Compared with those of bulk Bi2WO6 and Bi2WO6s, the diffraction angles of C-Bi2WO6s showed no changes, thereby indicating that carbon doping does not change the crystalline phase of Bi2WO6 [25]. The XRD peaks of Bi2WO6s and C-Bi2WO6s were weaker and broader compared with those of bulk Bi2WO6, which could be attributed to the thinner layered structure of sheet-like Bi2WO6 [23]. According to the Scherrer equation, a smaller grain size results in broader XRD peaks.
Field emission scanning electron microscopy (Fig. 2) and transmission electron microscopy (TEM; Fig. 3) were performed to investigate the microstructures of the samples. Interestingly, compared with bulk Bi2WO6, both Bi2WO6s and C-Bi2WO6s showed ultra-thin nanosheet structures, but the latter has small deformation, which could be attributed to the calcination treatment. Furthermore, this nanosheet-like structure of the samples was further confirmed by TEM and atomic force microscopy (AFM). The TEM micrographs in Fig. 3a and b reveal nearly transparent features, which suggests an ultra-thin nanosheet structure. The high-resolution TEM micrographs also demonstrated marked interplanar spacings of (200) and (020) planes, which correspond to the orientation of the Bi2WO6 nanosheet along the [001] projection [17]. The AFM image of C-Bi2WO6s shown in Fig. 3c reveals an average nanosheet thickness of ~ 4.1 nm, which corresponds to approximately three layers of Bi2WO6. These results demonstrate the intercalation of CTAB during the formation of the ultra-thin Bi2WO6 nanosheets. As a long carbon chain surfactant, CTAB could intercalate into the interlayers of Bi2WO6, thereby inducing the formation of ultra-thin Bi2WO6 nanosheets. Such an ultra-thin nanosheet structure could increase the availability of active sites and shorten the migration distance of charge carriers, thereby achieving effective charge separation [16, 26].
Energy-dispersive spectrometry (EDS) and elemental mapping were conducted to clarify the elemental composition of the C-Bi2WO6s catalyst. The EDS spectrum provided in Fig. 4 demonstrates that the elements Bi, W, C, and O are uniformly distributed in the C-Bi2WO6s catalyst. To further clarify the carbon content in the C-Bi2WO6s catalyst, C elemental analysis was carried out using an elemental analyzer. The elemental analysis result revealed that the atomic composition of C was about 0.36%.
X-Ray Photoelectron Spectroscopic Analysis
X-ray photoelectron spectroscopy (XPS) was performed on Bi2WO6s and C-Bi2WO6s, as shown in Fig. 5, to confirm the successful doping of carbon into Bi2WO6s. The survey XPS spectra confirmed the existence of Bi, W, O, and C in Bi2WO6s and C-Bi2WO6s. The high-resolution spectrum of Bi could be fitted to two peaks at 164.26 and 158.96 eV, which, respectively, correspond to Bi 4f5/2 and Bi 4f7/2 in Bi3+ [27]. After carbon doping, the binding energy of Bi shifted toward higher energy, thereby indicating the presence of Bi–C interactions. The binding energies of W at 37.36 and 35.26 eV in the XPS spectrum of Bi2WO6s correspond to the W6+ oxidation state. The binding energy of W also shifted toward higher binding energy, thus suggesting the successful doping of C. The binding energy of O in Bi2WO6s could be fitted to two peaks at 529.9 and 531.51 eV, which correspond to Bi–O and W–O, respectively. The binding energy of O 1s in C-Bi2WO6s slightly shifted, and the peak at 533.06 eV could be attributed to physically adsorbed water. The binding energy shifts of Bi, W, and O reflect strong interactions between Bi2WO6 and C [27, 28]. The binding energies of C 1s centered at 284.8, 286.3, and 288.8 eV could be assigned to C–C sp2-hybridized carbon on surface, C–O, and COO bonds, respectively, thus indicating that carbon was successfully doped into the Bi2WO6 nanosheets.
N2 Adsorption–Desorption Isotherms
The curves of all Bi2WO6 samples were classified as type IV isotherms according to the Brunauer–Deming–Deming–Teller classification (Fig. 6) [29]. Moreover, the curves of all Bi2WO6 samples showed a type H3 hysteresis loop, suggests that slit-like pores formed due to the stacking and aggregation of plate-like particles, which is in agreement with the sheet-like morphology of Bi2WO6. The pore size distribution curves confirm the presence of mesopores and macropores in the Bi2WO6 samples. The Brunauer–Emmett–Teller (BET) surface areas of bulk Bi2WO6, Bi2WO6s, and C-Bi2WO6s were measured to be 15.5, 43.8, and 42.7 m2/g, respectively. Compared with that of Bi2WO6s, the specific surface area of C-Bi2WO6s showed no obvious change, thus suggesting that the microstructure of Bi2WO6s is well preserved after carbon doping by thermal treatment. These results collectively confirm the formation of ultra-thin nanosheet structures, which increase the BET surface area of the material and provide more active sites for greater photocatalytic activity.
CO2 Adsorption
The CO2 adsorption curves of the samples were measured and are shown in Fig. 7. Bi2WO6s and C-Bi2WO6s show similar CO2 adsorption performance, which is 4 times higher than that of the bulk Bi2WO6. This enhanced CO2 adsorption ability is mainly attributed to large specific surface area [16, 30]. The comparable CO2 adsorption capacity of the two nanosheet samples indicated that carbon doping does not significantly change the catalyst microstructure, which is consistent with the N2 adsorption–desorption results. Enhancements in CO2 adsorption capacity can reinforce the photocatalytic CO2 reduction activity of the prepared materials [31].
UV–Visible Diffuse Reflectance Spectra
Figure 8 shows the UV–Vis absorption spectra of bulk Bi2WO6, Bi2WO6s, and C-Bi2WO6s. The absorption edge of bulk Bi2WO6 was approximately 430 nm, which corresponds to a band gap of 2.88 eV. Moreover, the absorption edge of Bi2WO6s was blue-shifted compared with that of bulk Bi2WO6 because of the quantum size effect [32]. Compared with that of Bi2WO6s, the absorption edge of C-Bi2WO6s was red-shifted, thereby indicating uniform carbon doping. Extension of the absorption tail was also observed in C-Bi2WO6s, which suggests that doping-related mid-gap states are generated and that these states regulate the band structure of Bi2WO6s. C-Bi2WO6s showed enhanced light absorption in the full-range visible-light region, which implies that, besides the lattice, carbon is also doped on the surface of Bi2WO6. These results indicate that carbon doping could reduce the band gap of Bi2WO6 nanosheets and enhance their light absorption [33].
Mott–Schottky Curves and Band Structure
Mott–Schottky plots were constructed, as shown in Fig. 9a–c, to confirm the band structures of the samples. All of the samples are typical n-type semiconductors, as evidenced by their positive slopes [34]. The flat-band potentials of bulk Bi2WO6, Bi2WO6s, and C-Bi2WO6s were − 1.08, − 1.30, and − 1.11 V (vs. Ag/AgCl, pH = 7), respectively. A more negative conduction band (CB) compared with that of bulk Bi2WO6 implies that the reduction ability of electrons is enhanced [35]. The more positive CB potential of C-Bi2WO6s compared with that of Bi2WO6s may be attributed to carbon doping [36]. The corresponding band structures of bulk Bi2WO6, Bi2WO6s, and C-Bi2WO6s are shown in Fig. 9d. The CB of the Bi2WO6 photocatalyst was more negative than the potential of the CO2/reduced-product redox couple and, thus, could provide sufficient driving force for CO2 reduction [33, 37, 38].
Photocatalytic CO2 Reduction and In-Situ FTIR Analysis
The photocatalytic CO2 reduction performance of all samples was measured under full-spectrum irradiation. As shown in Fig. 10, the generation rates of CH3OH and C2H5OH for Bi2WO6s were remarkably enhanced by approximately 4.7 and 3.0 times, respectively, compared with those of bulk Bi2WO6. Compared with that of Bi2WO6s, the photocatalytic CO2 reduction activity of C-Bi2WO6s was further improved, and the corresponding generation rates of CH3OH and C2H5OH were 1.15 and 0.65 μmol/(g·h), respectively. This photocatalytic performance is comparable with those of other bismuth-based photocatalysts (Table S1).
The photocatalytic reduction of CO2 over C-Bi2WO6s was investigated by in-situ Fourier transform infrared (FTIR) spectroscopy. As shown in Fig. 11, the peak at 1648 cm−1 can be attributed to H2O [39]. The peaks at 1684, 1670, 1662, 1575, 1559, and 1256 cm−1 can be attributed to carboxylate (CO −2 ) [39,40,41,42]. The peaks at 1696, 1636, 1623, 1617, 1458, 1449, 1436, 1406, 1397, and 1227 cm−1 are ascribed to bicarbonate (HCO −3 ) [39, 40, 42,43,44,45,46]. The peaks at 1654, 1361, 1340, and 1318 cm−1 refer to bidentate carbonate (b-CO 2−3 ) [40, 43, 46]. The peaks at 1569, 1541, 1532, 1522, 1508, 1498, 1488, 1474, 1466, and 1388 cm−1 are attributed to monodentate carbonate (m-CO 2−3 ) [39,40,41, 43, 47,48,49]. After light irradiation, a number of new peaks were observed. The peaks at 1375 and 1318 cm−1 are assigned to formate [50]. The peak at 1419 cm−1 is related to formaldehyde (HCHO) [35], and the peak at 1162 cm−1 is ascribed to methoxyl groups (CH3OH) [31, 51]. The presence of these peaks, which indicate intermediate products, reveals that CO2 conversion over C-Bi2WO6s is a multi-electron reduction process.
Mechanism of Photocatalytic Performance Enhancement
The charge transfer and separation dynamics of bulk Bi2WO6, Bi2WO6s, and C-Bi2WO6s were studied by transient photocurrent response analysis and electrochemical impedance spectroscopy (EIS). The photocurrent intensity of C-Bi2WO6s was larger than those of bulk Bi2WO6 and Bi2WO6s (Fig. 12a), suggesting the best electron transfer efficiency and lowest charge carrier recombination rate of C-Bi2WO6s sample [52]. Anodic spikes were observed in all samples when the light was switched on because of the separation of photogenerated electrons and holes at the interface of Bi2WO6/electrolyte. A decrease in photocurrent was subsequently observed, because photogenerated holes tend to recombine with electrons instead of undergoing capture by reduced species in the electrolyte. The EIS Nyquist plots of all samples were also obtained, as shown in Fig. 12b. A smaller semicircular radius usually indicates lower charge-transfer resistance. Among the samples studied, C-Bi2WO6s showed the smallest arc radius, which suggests that charge transfer occurs fastest in this sample.
Conclusions
In summary, ultra-thin (~ 4.1 nm) carbon-doped Bi2WO6 nanosheets were prepared by a simple hydrothermal treatment and calcination method. The prepared Bi2WO6 nanosheets exhibited twofold enhanced photocatalytic activity for CO2 reduction compared with that of pristine ultra-thin Bi2WO6 nanosheets. This enhancement was attributed to the ultra-thin nanosheet structure and carbon doping. An ultra-thin nanosheet structure provides more active sites and shortens the diffusion distance of charge carriers, thereby suppressing charge recombination. Carbon doping extends the light absorption ability of the catalyst and promotes charge separation. This work highlights the importance of defect engineering in photocatalytic energy conversion and provides new insights for fabricating efficient two-dimensional photocatalysts.
References
Wang HJ, Wang YJ, Guo LJ et al (2020) Solar-heating boosted catalytic reduction of CO2 under full-solar spectrum. Chin J Catal 41(1):131–139
Qiu CH, Bai S, Cao WJ et al (2020) Tunable syngas synthesis from photocatalytic CO2 reduction under visible-light irradiation by interfacial engineering. Trans Tianjin Univ 26(5):352–361
Wang SB, Han X, Zhang YH et al (2021) Inside-and-out semiconductor engineering for CO2 photoreduction: from recent advances to new trends. Small Struct 2(1):2000061
Bie C, Yu H, Cheng B et al (2021) Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst. Adv Mater 33(9):2003521
Chen F, Ma ZY, Ye LQ et al (2020) Macroscopic spontaneous polarization and surface oxygen vacancies collaboratively boosting CO2 photoreduction on BiOIO3 single crystals. Adv Mater 32(11):1908350
Yang Y, Tan HY, Cheng B et al (2021) Near-infrared-responsive photocatalysts. Small Methods 5(4):2001042
Liu LZ, Huang HW, Chen F et al (2020) Cooperation of oxygen vacancies and 2D ultrathin structure promoting CO2 photoreduction performance of Bi4Ti3O12. Sci Bull 65(11):934–943
Hao L, Kang L, Huang H et al (2019) Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction. Adv Mater 31(25):1900546
Cao SW, Wang YJ, Zhu BC et al (2020) Enhanced photochemical CO2 reduction in the gas phase by graphdiyne. J Mater Chem A 8(16):7671–7676
Xu F, Meng K, Cheng B et al (2020) Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat Commun 11(1):4613
Xia Y, Cheng B, Fan JJ et al (2020) Near-infrared absorbing 2D/3D ZnIn2S4/N-doped graphene photocatalyst for highly efficient CO2 capture and photocatalytic reduction. Sci China Mater 63(4):552–565
Xia Y, Tian ZH, Heil T et al (2019) Highly selective CO2 capture and its direct photochemical conversion on ordered 2D/1D heterojunctions. Joule 3(11):2792–2805
Xie Q, He WM, Liu SW et al (2020) Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin J Catal 41(1):140–153
Xia Y, Yu JG (2020) Reaction: rational design of highly active photocatalysts for CO2 conversion. Chem 6(5):1039–1040
Li Y, Li X, Zhang HW et al (2020) Design and application of active sites in g-C3N4-based photocatalysts. J Mater Sci Technol 56:69–88
Liang L, Lei F, Gao S et al (2015) Single unit cell bismuth tungstate layers realizing robust solar CO2 reduction to methanol. Angew Chem Int Ed 54(47):13971–13974
Zhou Y, Zhang Y, Lin M et al (2015) Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat Commun 6:8340
Cheng HF, Huang BB, Liu YY et al (2012) An anion exchange approach to Bi2WO6 hollow microspheres with efficient visible light photocatalytic reduction of CO2 to methanol. Chem Commun 48(78):9729–9731
Huang HW, Zhou C, Jiao XC et al (2020) Subsurface defect engineering in single-unit-cell Bi2WO6 monolayers boosts solar-driven photocatalytic performance. ACS Catal 10(2):1439–1443
Zhang YL, Zhao YC, Xiong Z et al (2021) Elemental mercury removal by I−-doped Bi2WO6 with remarkable visible-light-driven photocatalytic oxidation. Appl Catal B Environ 282:119534
Dai WL, Yu JJ, Deng YQ et al (2017) Facile synthesis of MoS2/Bi2WO6 nanocomposites for enhanced CO2 photoreduction activity under visible light irradiation. Appl Surf Sci 403:230–239
Wang WL, Zhao WL, Zhang HC et al (2021) 2D/2D step-scheme α-Fe2O3/Bi2WO6 photocatalyst with efficient charge transfer for enhanced photo-Fenton catalytic activity. Chin J Catal 42(1):97–106
Cao SW, Shen BJ, Tong T et al (2018) 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv Funct Mater 28(21):1800136
Wang J, Heil T, Zhu BC et al (2020) A single Cu-center containing enzyme-mimic enabling full photosynthesis under CO2 reduction. ACS Nano 14(7):8584–8593
Li CM, Chen G, Sun JX et al (2016) Doping effect of phosphate in Bi2WO6 and universal improved photocatalytic activity for removing various pollutants in water. Appl Catal B Environ 188:39–47
Li H, Zhu BC, Cao SW et al (2020) Controlling defects in crystalline carbon nitride to optimize photocatalytic CO2 reduction. Chem Commun 56(42):5641–5644
Di J, Xia JX, Ge YP et al (2015) Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl Catal B Environ 168–169:51–61
Tian J, Sang YH, Yu GW et al (2013) A Bi2WO6-based hybrid photocatalyst with broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Adv Mater 25(36):5075–5080
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069
Li H, Li F, Yu J et al (2021) 2D/2D FeNi-LDH/g-C3N4 hybrid photocatalyst for enhanced CO2 photoreduction. Acta Phys-Chim Sin 37(8):2010073
Cao SW, Li Y, Zhu BC et al (2017) Facet effect of Pd cocatalyst on photocatalytic CO2 reduction over g-C3N4. J Catal 349:208–217
Li YF, Zhang M, Zhou L et al (2021) Recent advances in surface-modified g-C3N4-based photocatalysts for H2 production and CO2 reduction. Acta Phys-Chim Sin 37(6):2009030
Fu J, Zhu B, Jiang C et al (2017) Hierarchical porous O-doped g-C3N4 with enhanced photocatalytic CO2 reduction activity. Small 13(15):28160415
Xu HT, Xiao R, Huang JR et al (2021) In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin J Catal 42(1):107–114
Xia PF, Zhu BC, Yu JG et al (2017) Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J Mater Chem A 5(7):3230–3238
Dong G, Zhao K, Zhang L (2012) Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem Commun 48(49):6178–6180
Mao J, Peng TY, Zhang XH et al (2013) Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal Sci Technol 3(5):1253–1260
Mao J, Li K, Peng TY (2013) Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal Sci Technol 3(10):2481–2498
Liu LJ, Zhao HL, Andino JM et al (2012) Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal 2(8):1817–1828
Liu LJ, Jiang YQ, Zhao HL et al (2016) Engineering coexposed 001 and 101 facets in oxygen-deficient TiO2 nanocrystals for enhanced CO2 photoreduction under visible light. ACS Catal 6(2):1097–1108
Yang RQ, Fu YL, Zhang Y et al (2004) In situ DRIFT study of low-temperature methanol synthesis mechanism on Cu/ZnO catalysts from CO2-containing syngas using ethanol promoter. J Catal 228(1):23–35
Ye LQ, Mao J, Peng TY et al (2014) Opposite photocatalytic activity orders of low-index facets of anatase TiO2 for liquid phase dye degradation and gaseous phase CO2 photoreduction. Phys Chem Chem Phys 16(29):15675–15680
Liu LJ, Zhao CY, Xu JY et al (2015) Integrated CO2 capture and photocatalytic conversion by a hybrid adsorbent/photocatalyst material. Appl Catal B Environ 179:489–499
Wu WQ, Bhattacharyya K, Gray K et al (2013) Photoinduced reactions of surface-bound species on titania nanotubes and platinized titania nanotubes: an in situ FTIR study. J Phys Chem C 117(40):20643–20655
Baltrusaitis J, Schuttlefield J, Zeitler E et al (2011) Carbon dioxide adsorption on oxide nanoparticle surfaces. Chem Eng J 170(2–3):471–481
Collins SE, Baltanás MA, Bonivardi AL (2004) An infrared study of the intermediates of methanol synthesis from carbon dioxide over Pd/β-Ga2O3. J Catal 226(2):410–421
Zhao HL, Liu LJ, Andino JM et al (2013) Bicrystalline TiO2 with controllable anatase-brookite phase content for enhanced CO2 photoreduction to fuels. J Mater Chem A 1(28):8209–8216
Liu LJ, Zhao CY, Li Y (2012) Spontaneous dissociation of CO2 to CO on defective surface of Cu(I)/TiO2−x nanoparticles at room temperature. J Phys Chem C 116(14):7904–7912
Li K, Peng TY, Ying ZH et al (2016) Ag-loading on brookite TiO2 quasi nanocubes with exposed 2 1 0 and 0 0 1 facets: activity and selectivity of CO2 photoreduction to CO/CH4. Appl Catal B Environ 180:130–138
Liao LF, Wu WC, Chen CY et al (2001) Photooxidation of formic acid vs formate and ethanol vs ethoxy on TiO2 and effect of adsorbed water on the rates of formate and formic acid photooxidation. J Phys Chem B 105(32):7678–7685
Boccuzzi F, Chiorino A, Manzoli M (2003) FTIR study of methanol decomposition on gold catalyst for fuel cells. J Power Sources 118(1–2):304–310
Liu L, Qi YH, Hu JS et al (2015) Efficient visible-light photocatalytic hydrogen evolution and enhanced photostability of core@shell Cu2O@g-C3N4 octahedra. Appl Surf Sci 351:1146–1154
Acknowledgements
The authors acknowledge the financial support from the National Key Research and Development Program of China (No. 2018YFB1502001), the National Natural Science Foundation of China (Nos. 51922081, 21773179, 51961135303, 51932007 and U1705251).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Zhang, J., Yu, J. et al. Ultra-Thin Carbon-Doped Bi2WO6 Nanosheets for Enhanced Photocatalytic CO2 Reduction. Trans. Tianjin Univ. 27, 338–347 (2021). https://doi.org/10.1007/s12209-021-00289-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-021-00289-5