Abstract
Introduction
Ventilator-Induced lung injury (VILI) is a form of acute lung injury that is initiated or exacerbated by mechanical ventilation. The aging lung is also more susceptible to injury. Harmful mechanical stretch of the alveolar epithelium is a recognized mechanism of VILI, yet little is known about how mechanical stretch affects aged epithelial cells. Disruption to Endoplasmic Reticulum (ER) homeostasis results in a condition known as ER stress that leads to disruption of cellular homeostasis, apoptosis, and inflammation. ER stress is increased with aging and other pathological stimuli. We hypothesized that age and mechanical stretch increase alveolar epithelial cells’ proinflammatory responses that are mediated by ER stress. Furthermore, we believed that inhibition of this upstream mechanism with 4PBA, an ER stress reducer, alleviates subsequent inflammation and monocyte recruitment.
Methods
Type II alveolar epithelial cells (ATII) were harvested from C57Bl6/J mice 2 months (young) and 20 months (old) of age. The cells were cyclically stretched at 15% change in surface area for up to 24 h. Prior to stretch, groups were administered 4PBA or vehicle as a control.
Results
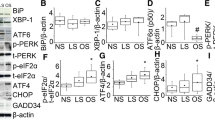
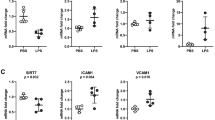
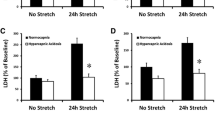
Mechanical stretch and age upregulated ER stress and proinflammatory MCP-1/CCL2 and MIP-1β/CCL4 chemokine expression in ATIIs. Age-matched and mismatched monocyte recruitment by ATII conditioned media was also quantified.
Conclusions
Age increases susceptibility to stretch-induced ER stress and downstream inflammatory gene expression in a primary ATII epithelial cell model. Administration of 4PBA attenuated the increased ER stress and proinflammatory responses from stretch and/or age and significantly reduced monocyte migration to ATII conditioned media.






Similar content being viewed by others
References
Andrade, P. V., J. M. dos Santos, H. C. A. Silva, D. D. Wilbert, S. S. Cavassani, and I. S. Oliveira-Júnior. Influence of hyperoxia and mechanical ventilation in lung inflammation and diaphragm function in aged versus adult rats. Inflammation 37:486–494, 2013.
Aoshiba, K., and A. Nagai. Chronic lung inflammation in aging mice. FEBS Lett. 581:3512–3516, 2007.
Benhamou, D., J. F. Muir, and B. Melen. Mechanical ventilation in elderly patients. Monaldi Arch. Chest Dis. Arch. Monaldi Mal Torace 53:547–551, 1998.
Bhandary, B., A. Marahatta, H.-R. Kim, and H.-J. Chae. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 14:434, 2013.
Bless, N. M., M. Huber-Lang, R.-F. Guo, R. L. Warner, H. Schmal, B. J. Czermak, T. P. Shanley, L. D. Crouch, A. B. Lentsch, V. Sarma, M. S. Mulligan, H. P. Friedl, and P. A. Ward. Role of CC chemokines (macrophage inflammatory protein-1β, monocyte chemoattractant protein-1, RANTES) in acute lung injury in rats. J. Immunol. 164:2650–2659, 2000.
Brown, M. K., and N. Naidoo. The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol. 3:263, 2012.
Brozzi, F., T. R. Nardelli, M. Lopes, I. Millard, J. Barthson, M. Igoillo-Esteve, F. A. Grieco, O. Villate, J. M. Oliveira, M. Casimir, M. Bugliani, F. Engin, G. S. Hotamisligil, P. Marchetti, and D. L. Eizirik. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 58:2307–2316, 2015.
Bystry, R. S., V. Aluvihare, K. A. Welch, M. Kallikourdis, and A. G. Betz. B cells and professional APCs recruit regulatory T cells via CCL4. Nat. Immunol. 2:1126–1132, 2001.
Canan, C. H., N. S. Gokhale, B. Carruthers, W. P. Lafuse, L. S. Schlesinger, J. B. Torrelles, and J. Turner. Characterization of lung inflammation and its impact on macrophage function in aging. J. Leukoc. Biol. 96:473–480, 2014.
Cavanaugh Jr., K. J., J. Oswari, and S. S. Margulies. Role of stretch on tight junction structure in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 25(5):584–591, 2012. https://doi.org/10.1165/ajrcmb.25.5.4486.
Chang, J., Y. Xia, K. Wasserloos, M. Deng, K. J. Blose, D. A. Vorp, H. R. Turnquist, T. R. Billiar, B. A. Pitt, M.-Z. Zhang, and L.-M. Zhang. Cyclic stretch induced IL-33 production through HMGB1/TLR-4 signaling pathway in murine respiratory epithelial cells. PLoS ONE 12:e0184770, 2017.
Chen, W., Y. Epshtein, X. Ni, R. O. Dull, A. E. Cress, J. G. N. Garcia, and J. R. Jacobson. Role of integrin β4 in lung endothelial cell inflammatory responses to mechanical stress. Sci. Rep. 5:16529, 2015.
Childs, L. M., M. Paskow, S. M. Morris, Jr, M. Hesse, and S. Strogatz. From inflammation to wound healing: using a simple model to understand the functional versatility of murine macrophages. Bull. Math. Biol. 73:2575–2604, 2011.
Cohen, T. S., K. J. Cavanaugh, and S. S. Margulies. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur. Respir. J. 32:854–861, 2008.
Corti, M., A. R. Brody, and J. H. Harrison. Isolation and primary culture of murine alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 14:309–315, 1996.
Corvol, H., F. Flamein, R. Epaud, A. Clement, and L. Guillot. Lung alveolar epithelium and interstitial lung disease. Int. J. Biochem. Cell Biol. 41:1643–1651, 2009.
Criswell, D. S., R. A. Shanely, J. J. Betters, M. J. McKenzie, J. E. Sellman, D. L. Van Gammeren, and S. K. Powers. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest 124:2302–2308, 2003.
Davidovich, N., B. C. DiPaolo, G. G. Lawrence, P. Chhour, N. Yehya, and S. S. Margulies. Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. Am. J. Respir. Cell Mol. Biol. 49:156–164, 2013.
Dolinay, D. T., D. B. E. Himes, M. M. Shumyatcher, M. G. G. Lawrence, and P. S. S. Margulies. Integrated stress response mediates epithelial injury in mechanical ventilation. Am. J. Respir. Cell Mol. Biol. 57(2):193–203, 2017. https://doi.org/10.1165/rcmb.2016-0404oc.
Endo, M., S. Oyadomari, M. Suga, M. Mori, and T. Gotoh. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J. Biochem. (Tokyo) 138:501–507, 2005.
Esteban, A., A. Anzueto, F. Frutos, I. Alía, L. Brochard, T. E. Stewart, S. Benito, S. K. Epstein, C. Apezteguía, P. Nightingale, A. C. Arroliga, M. J. Tobin, and Group for the MVIS. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287:345–355, 2002.
Fan, E., D. M. Needham, and T. E. Stewart. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA 294:2889–2896, 2005.
Feng, Y., Y. Amoateng-Adjepong, D. Kaufman, C. Gheorghe, and C. A. Manthous. Age, duration of mechanical ventilation, and outcomes of patients who are critically ill. Chest 136:759–764, 2009.
Franceschi, C., and J. Campisi. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69:S4–S9, 2014.
Frank, J. A., C. M. Wray, D. F. McAuley, R. Schwendener, and M. A. Matthay. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am. J. Physiol-Lung. Cell. Mol. Physiol. 291:L1191–L1198, 2006.
Freund, A., A. V. Orjalo, P.-Y. Desprez, and J. Campisi. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 16:238–246, 2010.
Garg, A. D., A. Kaczmarek, O. Krysko, P. Vandenabeele, D. V. Krysko, and P. Agostinis. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol. Med. 18:589–598, 2012.
Gibon, E., F. Loi, L. A. Córdova, J. Pajarinen, T. Lin, L. Lu, A. Nabeshima, Z. Yao, and S. B. Goodman. Aging affects bone marrow macrophage polarization: relevance to bone healing. Regen. Eng. Transl. Med. 2:98–104, 2016.
Goldman, J. L., S. Sammani, C. Kempf, L. Saadat, E. Letsiou, T. Wang, L. Moreno-Vinasco, A. N. Rizzo, J. D. Fortman, and J. G. N. Garcia. Pleiotropic effects of interleukin-6 in a “two-hit” murine model of acute respiratory distress syndrome. Pulm. Circ. 4:280, 2014.
Guo, R.-F., A. B. Lentsch, R. L. Warner, M. Huber-Lang, J. V. Sarma, T. Hlaing, M. M. Shi, N. W. Lukacs, and P. A. Ward. Regulatory effects of eotaxin on acute lung inflammatory injury. J. Immunol. 166:5208–5218, 2001.
Halbertsma, F. J. J., M. Vaneker, G. J. Scheffer, and J. G. van der Hoeven. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth. J. Med. 63:382–392, 2005.
Hawwa, R. L., M. A. Hokenson, Y. Wang, Z. Huang, S. Sharma, and J. Sanchez-Esteban. IL-10 inhibits inflammatory cytokines released by fetal mouse lung fibroblasts exposed to mechanical stretch. Pediatr. Pulmonol. 46:640, 2011.
Hegeman, M. A., S. N. T. Hemmes, M. T. Kuipers, L. D. J. Bos, G. Jongsma, J. J. T. H. Roelofs, K. F. van der Sluijs, N. P. Juffermans, M. B. Vroom, and M. J. Schultz. The extent of ventilator-induced lung injury in mice partly depends on duration of mechanical ventilation. Crit. Care Res. Pract. 2013.
Hegeman, M. A., M. P. Hennus, M. van Meurs, P. M. Cobelens, A. Kavelaars, N. J. Jansen, M. J. Schultz, A. J. van Vught, G. Molema, and C. J. Heijnen. Angiopoietin-1 treatment reduces inflammation but does not prevent ventilator-induced lung injury. PLoS ONE 5(12):e15653, 2010.
Heise, R. L., V. Stober, C. Cheluvaraju, J. W. Hollingsworth, and S. Garantziotis. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J. Biol. Chem. 286:17435–17444, 2011.
Helmerhorst, H. J. F., L. R. A. Schouten, G. T. M. Wagenaar, N. P. Juffermans, J. J. T. H. Roelofs, M. J. Schultz, E. de Jonge, and D. J. van Westerloo. Hyperoxia provokes a time- and dose-dependent inflammatory response in mechanically ventilated mice, irrespective of tidal volumes. Intensive Care Med. Exp. 5:27, 2017.
Herbert, J. A., M. S. Valentine, N. Saravanan, M. B. Schneck, R. Pidaparti, A. A. Fowler, A. M. Reynolds, and R. L. Heise. Conservative fluid management prevents age-associated ventilator induced mortality. Exp. Gerontol. 81:101–109, 2016.
Hibi, M., M. Murakami, M. Saito, T. Hirano, T. Taga, and T. Kishimoto. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63:1149–1157, 1990.
Irving, S. G., P. F. Zipfel, J. Balke, O. W. McBride, C. C. Morton, P. R. Burd, U. Siebenlist, and K. Kelly. Two inflammatory mediator cytokine genes are closely linked and variably amplified on chromosome 17q. Nucleic Acids Res. 18:3261–3270, 1990.
Jia, C.-E., D. Jiang, H. Dai, F. Xiao, and C. Wang. Pendrin, an anion exchanger on lung epithelial cells, could be a novel target for lipopolysaccharide-induced acute lung injury mice. Am. J. Transl. Res. 8:981, 2016.
Kim, H. J., J. S. Jeong, S. R. Kim, S. Y. Park, H. J. Chae, and Y. C. Lee. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-κB/HIF-1α signaling pathway. Sci. Rep. 3:1142, 2013.
Kolb, P. S., E. A. Ayaub, W. Zhou, V. Yum, J. G. Dickhout, and K. Ask. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 61:45–52, 2015.
Korfei, M., C. Ruppert, P. Mahavadi, I. Henneke, P. Markart, M. Koch, G. Lang, L. Fink, R.-M. Bohle, W. Seeger, T. E. Weaver, and A. Guenther. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 178:838–846, 2008.
Lee, A. S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35:373–381, 2005.
Levy, B. D., and A. M. K. Choi. Acute respiratory distress syndrome|clinical gate. Clincalgate 2015. http://clinicalgate.com/acute-respiratory-distress-syndrome-2/ [17 Apr. 2016]
Lloyd, C. Chemokines in allergic lung inflammation. Immunology 105:144, 2002.
Luh, S., and C. Chiang. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): the mechanism, present strategies and future perspectives of therapies. J. Zhejiang. Univ. Sci. B 8:60–69, 2007.
Maus, U., J. Huwe, R. Maus, W. Seeger, and J. Lohmeyer. Alveolar JE/MCP-1 and endotoxin synergize to provoke lung cytokine upregulation, sequential neutrophil and monocyte influx, and vascular leakage in mice. Am. J. Respir. Crit. Care Med. 164:406–411, 2001.
Moldoveanu, B., P. Otmishi, P. Jani, J. Walker, X. Sarmiento, J. Guardiola, M. Saad, and J. Yu. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2:1, 2009.
Mukai, S., Y. Ogawa, F. Urano, C. Kudo-Saito, Y. Kawakami, and K. Tsubota. Novel treatment of chronic graft-versus-host disease in mice using the ER stress reducer 4-phenylbutyric acid. Sci. Rep. 7:41939, 2017.
Müller, H. C., K. Hellwig, S. Rosseau, T. Tschernig, A. Schmiedl, B. Gutbier, B. Schmeck, S. Hippenstiel, H. Peters, L. Morawietz, N. Suttorp, and M. Witzenrath. Simvastatin attenuates ventilator-induced lung injury in mice. Crit. Care 14:R143, 2010.
Murray, M. Y., T. P. Birkland, J. D. Howe, A. D. Rowan, M. Fidock, W. C. Parks, and J. Gavrilovic. Macrophage migration and invasion is regulated by MMP10 expression. PLoS ONE 8:e63555, 2013.
Naidoo, N. ER and aging—protein folding and the ER stress response. Ageing Res. Rev. 8:150–159, 2009.
Network TARDS. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 342:1301–1308, 2000.
Nin, N., J. A. Lorente, M. Paula, P. Fernández-Segoviano, O. Peñuelas, A. Sánchez-Ferrer, L. Martínez-Caro, and A. Esteban. Aging increases the susceptibility to injurious mechanical ventilation. Intensiv Care Med. 34:923–931, 2008.
O’Dea, K. P., M. R. Wilson, J. O. Dokpesi, K. Wakabayashi, L. Tatton, N. van Rooijen, and M. Takata. Mobilization and Margination of Bone Marrow Gr-1high Monocytes during sub-clinical endotoxemia predisposes the lungs towards acute injury. J. Immunol. 1950(182):1155–1166, 2009.
O’Dea, K. P., A. J. Young, H. Yamamoto, J. L. Robotham, F. M. Brennan, and M. Takata. Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am. J. Respir. Crit. Care Med. 172:1119–1127, 2005.
Okada, M., A. Matsumori, K. Ono, Y. Furukawa, T. Shioi, A. Iwasaki, K. Matsushima, and S. Sasayama. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18:894–901, 1998.
Oslowski, C. M., and F. Urano. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 490:71–92, 2011.
Puchta, A., A. Naidoo, C. P. Verschoor, D. Loukov, N. Thevaranjan, T. S. Mandur, P. Nguyen, M. Jordana, M. Loeb, Z. Xing, L. Kobzik, M. J. Larché, and D. M. E. Bowdish. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog. 12:e1005368, 2016.
Rentzsch, I., C. L. Santos, R. Huhle, J. M. C. Ferreira, T. Koch, C. Schnabel, E. Koch, P. Pelosi, P. R. M. Rocco, and M. G. de Abreu. Variable stretch reduces the pro-inflammatory response of alveolar epithelial cells. PLoS ONE 12:e0182369, 2017.
Ruthman, C. A., and E. Festic. Emerging therapies for the prevention of acute respiratory distress syndrome. Ther. Adv. Respir. Dis. 9:173–187, 2015.
Schouten, L. R. A., M. J. Schultz, A. H. van Kaam, N. P. Juffermans, A. P. Bos, and R. M. Wösten-van Asperen. Association between maturation and aging and pulmonary responses in animal models of lung injury: a systematic review. Anesthesiology 123:389–408, 2015.
Shallo, H., T. P. Plackett, S. A. Heinrich, and E. J. Kovacs. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns 29:641–647, 2003.
Shao, L., D. Meng, F. Yang, H. Song, and D. Tang. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 487:194–200, 2017.
Shi, C., and E. G. Pamer. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11(11):762, 2011.
Skerrett, S. J., H. D. Liggitt, A. M. Hajjar, R. K. Ernst, S. I. Miller, and C. B. Wilson. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L143–L152, 2004.
Terragni, P. P., G. Rosboch, A. Tealdi, E. Corno, E. Menaldo, O. Davini, G. Gandini, P. Herrmann, L. Mascia, M. Quintel, A. S. Slutsky, L. Gattinoni, and V. M. Ranieri. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 175(2):160–166, 2012. https://doi.org/10.1164/rccm.200607-915oc.
Tremblay, L. N., D. Miatto, Q. Hamid, A. Govindarajan, and A. S. Slutsky. Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit. Care Med. 30:1693–1700, 2002.
Trouplin, V., N. Boucherit, L. Gorvel, F. Conti, G. Mottola, and E. Ghigo. Bone marrow-derived macrophage production. J. Vis. Exp. JoVE 2013
van Zoelen, M. A. D., M. I. Verstege, C. Draing, R. deBeer, C. van’t Veer, S. Florquin, P. Bresser, J. S. van der Zee, A. A. te Velde, S. von Aulock, and T. van der Poll. Endogenous MCP-1 promotes lung inflammation induced by LPS and LTA. Mol. Immunol. 48:1468–1476, 2011.
Vlahakis, N. E., M. A. Schroeder, A. H. Limper, and R. D. Hubmayr. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 277:L167–L173, 1999.
Wang, J. M., B. Sherry, M. J. Fivash, D. J. Kelvin, and J. J. Oppenheim. Human recombinant macrophage inflammatory protein-1 alpha and -beta and monocyte chemotactic and activating factor utilize common and unique receptors on human monocytes. J. Immunol. 150:3022–3029, 1993.
Wilson, M. R., K. P. O’Dea, D. Zhang, A. D. Shearman, N. van Rooijen, and M. Takata. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 179:914–922, 2009.
Wolters, P. J., C. Wray, R. E. Sutherland, S. S. Kim, J. Koff, Y. Mao, and J. A. Frank. Neutrophil-derived il-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J Immunol 1950(182):8056–8062, 2009.
Wung, B. S., J. J. Cheng, Y. J. Chao, J. Lin, Y. J. Shyy, and D. L. Wang. Cyclical strain increases monocyte chemotactic protein-1 secretion in human endothelial cells. Am. J. Physiol.-Heart. Circ. Physiol. 270:H1462–H1468, 1996.
Xia, S., X. Zhang, S. Zheng, R. Khanabdali, B. Kalionis, J. Wu, W. Wan, and X. Tai. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J Immunol Res 2016
Xiao, C., A. Giacca, and G. F. Lewis. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and β-cell dysfunction in humans. Diabetes 60:918, 2011.
Yin, L., D. Zheng, G. V. Limmon, N. H. Leung, S. Xu, J. C. Rajapakse, H. Yu, V. T. Chow, and J. Chen. Aging exacerbates damage and delays repair of alveolar epithelia following influenza viral pneumonia. Respir. Res. 15(1):116, 2014.
Yuan, R., L. L. Peters, and B. Paigen. Mice as a mammalian model for research on the genetics of aging. ILAR J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 52:4–15, 2011.
Zhong, Q., B. Zhou, D. K. Ann, P. Minoo, Y. Liu, A. Banfalvi, M. S. Krishnaveni, M. Dubourd, L. Demaio, B. C. Willis, K.-J. Kim, R. M. duBois, E. D. Crandall, M. F. Beers, and Z. Borok. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 45:498, 2011.
Zhu, J.-P., K. Wu, J.-Y. Li, Y. Guan, Y.-H. Sun, W.-J. Ma, and Q.-M. Xie. Cryptoporus volvatus polysaccharides attenuate LPS-induced expression of pro-inflammatory factors via the TLR2 signaling pathway in human alveolar epithelial cells. Pharm. Biol. 54(2):347–353, 2016.
Acknowledgments
The authors acknowledge the assistance of Niraja Bohidar in aiding in cell isolation experiments.
Funding
This study was funded by the National Institutes of Health (R01AG041823) and the National Science Foundation (CMMI-1351162).
Conflict of interest
MSV, PAL, JAH, FKG, MBS, SK, JN, AMR, and RLH declares that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kris Noel Dahl oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valentine, M.S., Link, P.A., Herbert, J.A. et al. Inflammation and Monocyte Recruitment Due to Aging and Mechanical Stretch in Alveolar Epithelium are Inhibited by the Molecular Chaperone 4-Phenylbutyrate. Cel. Mol. Bioeng. 11, 495–508 (2018). https://doi.org/10.1007/s12195-018-0537-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-018-0537-8