Abstract
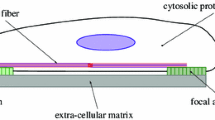
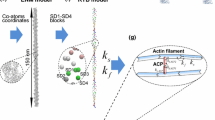
Actin stress fibers (SFs) in live cells consist of series of dynamic individual sarcomeric units. Within a group of consecutive SF sarcomeres, individual sarcomeres can spontaneously shorten or lengthen without changing the overall length of this group, but the underlying mechanism is unclear. We used a computational model to test our hypothesis that this dynamic behavior is inherent to the heterogeneous mechanical properties of the sarcomeres and the cytoplasmic viscosity. Each sarcomere was modeled as a discrete element consisting of an elastic spring, a viscous dashpot and an active contractile unit all connected in parallel, and experiences forces as a result of actin filament elastic stiffness, myosin II contractility, internal viscoelasticity, or cytoplasmic drag. When all four types of forces are considered, the simulated dynamic behavior closely resembles the experimental observations, which include a low-frequency fluctuation in individual sarcomere length and compensatory lengthening and shortening of adjacent sarcomeres. Our results suggest that heterogeneous stiffness and viscoelasticity of actin fibers, heterogeneous myosin II contractility, and the cytoplasmic drag are sufficient to cause spontaneous fluctuations in SF sarcomere length. Our results shed new light to the dynamic behavior of SF and help design experiments to further our understanding of SF dynamics.







Similar content being viewed by others
References
Amato, P. A., and D. L. Taylor. Probing the mechanism of incorporation of fluorescently labeled actin into stress fibers. J. Cell Biol. 102:1074–1084, 1986.
Besser, A. S. & Schwarz, U. S. Coupling biochemistry and mechanics in cell adhesion: a model for inhomogeneous stress fiber contraction. J. New Phys. 9:425 (2007).
Burridge, K., and J. R. Feramisco. Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature 294:565–567, 1981.
Burridge, K., and E. S. Wittchen. The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 200:9–19, 2013.
Cai, Y., et al. Cytoskeletal coherence requires myosin-IIA contractility. J. Cell Sci. 123:413–423, 2010.
Cavnar, P. J., S. G. Olenych, and T. C. Keller, 3rd. Molecular identification and localization of cellular titin, a novel titin isoform in the fibroblast stress fiber. Cell Motil Cytoskeleton 64:418–433, 2007.
Chapin, L. M., E. Blankman, M. A. Smith, Y. T. Shiu, and M. C. Beckerle. Lateral communication between stress fiber sarcomeres facilitates a local remodeling response. Biophys. J. 103:2082–2092, 2012.
Charras, G. T., and M. A. Horton. Determination of cellular strains by combined atomic force microscopy and finite element modeling. Biophys. J. 83:858–879, 2002.
Colombelli, J., et al. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 122:1665–1679, 2009.
Cramer, L. P., M. Siebert, and T. J. Mitchison. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J. Cell Biol. 136:1287–1305, 1997.
Decker, B., and M. S. Kellermayer. Periodically arranged interactions within the myosin filament backbone revealed by mechanical unzipping. J. Mol. Biol. 377:307–310, 2008.
Deguchi, S., T. Ohashi, and M. Sato. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 39:2603–2610, 2006.
Discher, D. E., P. Janmey, and Y. L. Wang. Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143, 2005.
Endlich, N., C. A. Otey, W. Kriz, and K. Endlich. Movement of stress fibers away from focal adhesions identifies focal adhesions as sites of stress fiber assembly in stationary cells. Cell Motil Cytoskeleton 64:966–976, 2007.
Esue, O., Y. Tseng, and D. Wirtz. Alpha-actinin and filamin cooperatively enhance the stiffness of actin filament networks. PLoS ONE 4:e4411, 2009.
Frank, D., and N. Frey. Cardiac Z-disc signaling network. J. Biol. Chem. 286:9897–9904, 2011.
Gautel, M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Arch. 462:119–134, 2011.
Gordon, A. M., A. F. Huxley, and F. J. Julian. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184:170–192, 1966.
Guolla, L., M. Bertrand, K. Haase, and A. E. Pelling. Force transduction and strain dynamics in actin stress fibres in response to nanonewton forces. J. Cell Sci. 125:603–613, 2012.
Haga, H., et al. Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy 82:253–258, 2000.
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279:509–514, 1998.
Hill, A. V. Length of muscle, and the heat and tension developed in an isometric contraction. J. Physiol. 60:237–263, 1925.
Hoffman, L. M., et al. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J. Cell Biol. 172:771–782, 2006.
Hotulainen, P., and P. Lappalainen. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 173:383–394, 2006.
Isenberg, G., P. C. Rathke, N. Hulsmann, W. W. Franke, and K. E. Wohlfarth-Bottermann. Cytoplasmic actomyosin fibrils in tissue culture cells: direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser micro-beam dissection. Cell Tissue Res. 166:427–443, 1976.
Jaffe, A. B., and A. Hall. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247–269, 2005.
Katoh, K., Y. Kano, M. Masuda, H. Onishi, and K. Fujiwara. Isolation and contraction of the stress fiber. Mol. Biol. Cell 9:1919–1938, 1998.
Kaunas, R., H.-J. Hsu, and S. Deguchi. Sarcomeric model of stretch-induced stress fiber reorganization. Cell Health and Cytoskeleton 3:13–22, 2011.
Kreis, T. E., K. H. Winterhalter, and W. Birchmeier. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc. Natl Acad Sci. U.S.A. 76:3814–3818, 1979.
Kumar, S., et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J . 90:3762–3773, 2006.
Lazarides, E., and K. Burridge. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell 6:289–298, 1975.
Peterson, L. J., et al. Simultaneous stretching and contraction of stress fibers in vivo. Mol. Biol. Cell 15:3497–3508, 2004.
Ponti, A., et al. Periodic patterns of actin turnover in lamellipodia and lamellae of migrating epithelial cells analyzed by quantitative Fluorescent Speckle Microscopy. Biophys. J. 89:3456–3469, 2005.
Rassier, D. E. The mechanisms of the residual force enhancement after stretch of skeletal muscle: non-uniformity in half-sarcomeres and stiffness of titin. Proc. Biol. Sci. 279:2705–2713, 2012.
Reedy, M. K., G. F. Baht, and D. A. Fishman. How many myosins per cross-bridge? I. Flight muscle myofibrils from the blowfly, Sarcophaga bullata. Cold Spring Harbor Symposia Quant. Biol. 37:397–421 (1973).
Rossier, O. M., et al. Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 29:1055–1068, 2010.
Russell, B., M. W. Curtis, Y. E. Koshman, and A. M. Samarel. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J. Mol. Cell. Cardiol. 48:817–823, 2010.
Russell, R. J., S. L. Xia, R. B. Dickinson, and T. P. Lele. Sarcomere mechanics in capillary endothelial cells. Biophys. J. 97:1578–1585, 2009.
Satcher, Jr., R. L., and C. F. Dewey, Jr. Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton. Biophys. J. 71:109–118, 1996.
Schillers, H., M. Walte, K. Urbanova, and H. Oberleithner. Real-time monitoring of cell elasticity reveals oscillating myosin activity. Biophys. J. 99:3639–3646, 2010.
Schoenberg, R. J. P. a. M. Force generation and shortening in skeletal muscle. Comprehensive Physiology Supplement 27: Handbook of Physiology, Skeletal Muscle, 173–187, 2011.
Smith, M. A., et al. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell 19:365–376, 2010.
Stachowiak, M. R., and B. O’Shaughnessy. Recoil after severing reveals stress fiber contraction mechanisms. Biophys. J. 97:462–471, 2009.
Turnacioglu, K. K., J. W. Sanger, and J. M. Sanger. Sites of monomeric actin incorporation in living PtK2 and REF-52 cells. Cell Motil Cytoskeleton 40:59–70, 1998.
Vogel, V., and M. Sheetz. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7:265–275, 2006.
Wang, N., et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl Acad Sci. U.S.A. 98:7765–7770, 2001.
Weber, K., and U. Groeschel-Stewart. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc. Natl Acad. Sci. U.S.A. 71:4561–4564, 1974.
Yam, P. T., and J. A. Theriot. Repeated cycles of rapid actin assembly and disassembly on epithelial cell phagosomes. Mol. Biol. Cell 15:5647–5658, 2004.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R01GM50877), the Huntsman Cancer Foundation, and shared resources from the Cancer Center Support Grant (2 P30 CA42014-21) to MCB. YTS is supported in part by grants from the National Institutes of Health (R01HL67646 and R01DK088777).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Chapin, L.M., Edgar, L.T., Blankman, E. et al. Mathematical Modeling of the Dynamic Mechanical Behavior of Neighboring Sarcomeres in Actin Stress Fibers. Cel. Mol. Bioeng. 7, 73–85 (2014). https://doi.org/10.1007/s12195-013-0318-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-013-0318-3