Abstract
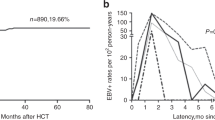
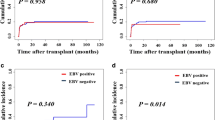
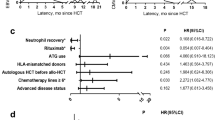
The present study was conducted to investigate Epstein-Barr virus (EBV) reactivation after hematopoietic cell transplantation (HCT) in Korean children living in an area of a high seroprevalence against EBV and to determine the impact of recipient age on EBV reactivation. Medical records of 248 children and adolescents who had received allogeneic HCT were retrospectively reviewed. The trends of EBV reactivation and post-transplant lymphoproliferative disorders (PTLDs) were evaluated and compared between younger (≤10 years old) and older (11–20 years old) groups. EBV reactivation occurred in 177 cases (71.4 %) and high-level EBV reactivation, defined as a virus DNA titer of 300,000 copies/mL or higher, occurred in 21 cases (8.5 %). PTLD was diagnosed in five cases (2.0 %), and one of these patients died. The EBV reactivation rate was not significantly different between the two age groups; however, high-level reactivation and PTLD were more significantly frequent in the older than in the younger group (P = 0.030 and P = 0.026, respectively). In conclusion, older children and adolescents are more likely to experience high-level EBV reactivation and PTLDs, and higher EBV DNA titers than those previously reported may be a predictor of PTLD in areas with a high seroprevalence against EBV.






Similar content being viewed by others
References
Odumade OA, Hogquist KA, Balfour HH Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011;24:193–209.
Rowe DT, Webber S, Schauer EM, Reyes J, Green M. Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis. 2001;3:79–87.
Landais E, Saulquin X, Houssaint E. The human T cell immune response to Epstein-Barr virus. Int J Dev Biol. 2005;49:285–92.
Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010;23:350–66.
Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002–8.
Omar H, Hagglund H, Gustafsson-Jernberg A, LeBlanc K, Mattsson J, Remberger M, et al. Targeted monitoring of patients at high risk of post-transplant lymphoproliferative disease by quantitative Epstein-Barr virus polymerase chain reaction. Transpl Infect Dis. 2009;11:393–9.
Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Kim HJ, et al. Clinical characteristics and outcomes of posttransplant lymphoproliferative disorders following allogeneic hematopoietic stem cell transplantation in Korea. J Korean Med Sci. 2006;21:259–64.
Sundin M, Le Blanc K, Ringden O, Barkholt L, Omazic B, Lergin C, et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91:1059–67.
Ocheni S, Kroeger N, Zabelina T, Sobottka I, Ayuk F, Wolschke C, et al. EBV reactivation and post transplant lymphoproliferative disorders following allogeneic SCT. Bone Marrow Transplant. 2008;42:181–6.
Oh SH, Lee YA, Moon WY, Ko TS, Park YS, Moon HN, et al. Prevalence of Epstein-Barr virus (EBV) antibody in Korean children. J Korean Pediatr Soc. 1994;37:804–11.
Takeuchi K, Tanaka-Taya K, Kazuyama Y, Ito YM, Hashimoto S, Fukayama M, et al. Prevalence of Epstein-Barr virus in Japan: trends and future prediction. Pathol Int. 2006;56:112–6.
Hoshino Y, Kimura H, Tanaka N, Tsuge I, Kudo K, Horibe K, et al. Prospective monitoring of the Epstein-Barr virus DNA by a real-time quantitative polymerase chain reaction after allogenic stem cell transplantation. Br J Haematol. 2001;115:105–11.
Chen DB, Song QJ, Chen YX, Chen YH, Shen DH. Clinicopathologic spectrum and EBV status of post-transplant lymphoproliferative disorders after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2013;97:117–24.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1994;15:825–8.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–70.
Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 1999;94:3294–306.
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–9.
Clave E, Agbalika F, Bajzik V, de Latour Peffault R, Trillard M, Rabian C, et al. Epstein-Barr virus (EBV) reactivation in allogeneic stem-cell transplantation: relationship between viral load, EBV-specific T-cell reconstitution and rituximab therapy. Transplantation. 2004;77:76–84.
Worth A, Conyers R, Cohen J, Jagani M, Chiesa R, Rao K, et al. Pre-emptive rituximab based on viraemia and T cell reconstitution: a highly effective strategy for the prevention of Epstein-Barr virus-associated lymphoproliferative disease following stem cell transplantation. Br J Haematol. 2011;155:377–85.
van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood. 2001;98:972–8.
Greenfield HM, Gharib MI, Turner AJ, Guiver M, Carr T, Will AM, et al. The impact of monitoring Epstein-Barr virus PCR in paediatric bone marrow transplant patients: can it successfully predict outcome and guide intervention? Pediatr Blood Cancer. 2006;47:200–5.
Bordon V, Padalko E, Benoit Y, Dhooge C, Laureys G. Incidence, kinetics, and risk factors of Epstein-Barr virus viremia in pediatric patients after allogeneic stem cell transplantation. Pediatr Transplant. 2012;16:144–50.
Patriarca F, Medeot M, Isola M, Battista ML, Sperotto A, Pipan C, et al. Prognostic factors and outcome of Epstein-Barr virus DNAemia in high-risk recipients of allogeneic stem cell transplantation treated with preemptive rituximab. Transpl Infect Dis. 2013;15:259–67.
Green M, Michaels MG. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am J Transplant. 2013;13(Suppl 3):41–54.
Opelz G, Daniel V, Naujokat C, Döhler B. Epidemiology of pretransplant EBV and CMV serostatus in relation to posttransplant non-Hodgkin lymphoma. Transplantation. 2009;88:962–7.
Chan TS, Hwang YY, Gill H, Au WY, Leung AY, Tse E, et al. Post-transplant lymphoproliferative diseases in Asian solid organ transplant recipients: late onset and favorable response to treatment. Clin Transplant. 2012;26:679–83.
Kullberg-Lindh C, Mellgren K, Friman V, Fasth A, Ascher H, Nilsson S, et al. Opportunistic virus DNA levels after pediatric stem cell transplantation: serostatus matching, anti-thymocyte globulin, and total body irradiation are additive risk factors. Transpl Infect Dis. 2011;13:122–30.
Schönberger S, Meisel R, Adams O, Pufal Y, Laws HJ, Enczmann J, et al. Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1428–35.
van Tol MJ, Gerritsen EJ, de Lange GG, van Leeuwen AM, Jol-van der Zijde CM, Oudeman-Gruber NJ, et al. The origin of IgG production and homogeneous IgG components after allogeneic bone marrow transplantation. Blood. 1996;87:818–26.
Yamazaki R, Nakasone H, Tanaka Y, Sato M, Terasako K, Wada H, et al. Allotype analysis to distinguish the origin of varicella-zoster virus immunoglobulin G after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1013–20.
Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol. 2007;8:212–8.
Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N, et al. Impaired recovery of Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood. 2003;101:4290–7.
Chakrabarti S, Milligan DW, Pillay D, Mackinnon S, Holder K, Kaur N, et al. Reconstitution of the Epstein-Barr virus-specific cytotoxic T-lymphocyte response following T-cell-depleted myeloablative and nonmyeloablative allogeneic stem cell transplantation. Blood. 2003;102:839–42.
Hoegh-Petersen M, Goodyear D, Geddes MN, Liu S, Ugarte-Torres A, Liu Y, et al. High incidence of post transplant lymphoproliferative disorder after antithymocyte globulin-based conditioning and ineffective prediction by day 28 EBV-specific T lymphocyte counts. Bone Marrow Transplant. 2011;46:1104–12.
Castagnola E, Cappelli B, Erba D, Rabagliati A, Lanino E, Dini G. Cytomegalovirus infection after bone marrow transplantation in children. Hum Immunol. 2004;65:412–22.
Zallio F, Primon V, Tamiazzo S, Pini M, Baraldi A, Corsetti MT, et al. Epstein-Barr virus reactivation in allogeneic stem cell transplantation is highly related to cytomegalovirus reactivation. Clin Transplant. 2013;27:E491–7.
Conflict of interest
There is no conflict of interest for all authors.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Han, S.B., Bae, E.Y., Lee, J.W. et al. Features of Epstein-Barr virus reactivation after allogeneic hematopoietic cell transplantation in Korean children living in an area of high seroprevalence against Epstein-Barr virus. Int J Hematol 100, 188–199 (2014). https://doi.org/10.1007/s12185-014-1613-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-014-1613-z