Abstract
Seventy to 80 % of patients with acute myeloid leukemia (AML) achieve complete remission following intensive chemotherapy, but more than 50 % of patients in remission subsequently relapse, which is often associated with clinical drug resistance. Therapy based on monoclonal antibodies (mAbs) has been developed to increase the selectivity of cytotoxic agents by conjugating them with a mAb. Gemtuzumab ozogamicin (GO) is a conjugate of a cytotoxic agent, a calicheamicin derivative, linked to a recombinant humanized mAb directed against the CD33 antigen, which is expressed on leukemia cells from more than 90 % of patients with AML. This conjugated mAb was introduced following promising results from phase I and II studies. However, the initial phase III study did not confirm the efficacy of GO in combination with conventional chemotherapies. Several subsequent phase III studies have shown the efficacy of GO in favorable and intermediate risk AML. Several resistance mechanisms against GO have been reported. Multidrug resistant (MDR) P-glycoprotein (P-gp), a trans-membrane glycoprotein that pumps out many anti-leukemic agents from cells, also affects GO. For this reasons, GO has been used in combination with MDR modifiers, such as cyclosporine, and in cases without P-gp. Several investigators have reported successful results of the use of GO in acute promyelocytic leukemia (APL). GO has also been described as effective in cases relapsed after treatment with all-trans retinoic acid (ATRA), arsenic acid and conventional chemotherapeutic agents. The efficacy of GO will be studied mainly in a favorable risk of AML, such as core binding factor leukemia and APL. In addition, suitable combinations with other chemotherapies and administration schedules should be discussed.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is one of the most prevalent hematological malignancies [1]. It is characterized by the proliferation of clonal hematopoietic precursor cells and impairment of normal hematopoiesis. Many agents have been introduced in the treatment of AML, and 60–80 % of AML cases achieve remission [2, 3]. However, a considerable number of patients relapse, and as a result, disease-free survival (DFS) remains at around 20 % [2]. Recent progress in the molecular analysis of AML has led to molecular-targeted therapies [4, 5]. Monoclonal antibody therapy against CD33 also emerged from advances in molecular biology.
Gemtuzumab ozogamicin (GO), development code CMA676, is a conjugate of a calicheamicin derivative and a recombinant humanized antibody (IgG4) directed against the CD33 antigen [6]. Calicheamicin is a highly potent anti-tumor antibiotic [7–10], which binds to DNA, breaks double-stranded DNA, and induces cell death. Extensive basic and clinical results relating to this agent have been reported, and its characteristics and efficacy have been demonstrated over time.
CD33
The CD33 antigen, a 67-kDa trans-membrane glycoprotein, belongs to the immunoglobulin gene superfamily of sialic acid-binding immunoglobulin (Ig)-like lectins (siglecs) [6, 11, 12]. It consists of two Ig-like extracellular domains and two cytoplasmic domains [13]. Although the precise function of CD33 has not been elucidated, it is thought to be related to cell adhesion and interaction. CD33 suppresses cell proliferation and function, and induces apoptosis in vitro [14], but it remains unclear whether CD33 also exerts these inhibitory functions in vivo.
In normal hematopoiesis, CD33 is expressed on myelocyte and myelomonocytic precursor cells, as well as mature myeloid lineage cells, macrophages, monocytes, and dendritic cells [15–17]. The amount of CD33 peaks in promyelocytes and myelocytes, and is downregulated with maturation of the myeloid lineage. CD33 is also expressed on erythroblasts, megakaryoblasts, and Kupffer cells [11, 12], but not on normal hematopoietic stem cells [18, 19]. Therefore, CD33 is considered to be a useful target for the development of therapeutic agents against AML.
Previous reports have suggested that 65–90 % of AML is CD33-positive. The variation may derive from methodological differences or definitions of what constitutes CD33-positive [17, 20–23]. The amount of CD33 on AML cells is estimated at 10,000–20,000 copies/cell [24]. CD33 is sometimes determined on acute lymphoblastic leukemia (ALL), but the amount is relatively small [25]. The molecular differences of CD33 between AML and ALL cells remain unclear [26].
Since CD33 is rapidly internalized after antibody binding, antibody-cytotoxic agent complexes can effectively be taken up by leukemia cells. Radio- and toxin-labeled anti-CD33 antibodies have been developed, including conjugates of radioisotopes, calicheamicin, gelonin, and ricin [27–30]. Of these, GO has shown the most encouraging results [31].
The effect of GO on leukemia cells in bone marrows is reportedly influenced by the amount of CD33 antigen in the peripheral blood [32]. GO may thus be lost to some extent in the circulation before it reaches the bone marrow. This suggests that GO might be made more effective by the reduction of CD33 in peripheral blood by chemotherapy [33].
CD34 is often co-expressed on CD33-positive AML cells [24]. In our study, GO was less effective on CD34-positive leukemia cells, even when they expressed a sufficient amount of CD33; this effect was independent of the amount of CD34 [34]. Sievers et al. [35] reported that expression of CD34 was associated with shorter survival after treatment with GO. It remains to be determined why GO is less effective on CD34-positive cells. One reason may be that CD34-positive cells express more P-glycoprotein (P-gp) than do CD34-negative cells.
Pharmacology
GO is a humanized IgG4 anti-CD33 monoclonal antibody (hP67.6) conjugated to NAc-gamma calicheamicin DMH, a hydrazide derivative of calicheamicin [36]. Approximately, half of antibodies are linked to calicheamicin, with an average load of several molecules of calicheamicin per antibody, while others are not. After GO binds to CD33 on the cells, CD33-antibody complexes are internalized and transferred into lysosomes [37]. The calicheamicin derivative is released via hydrolysis in the acid environment of the lysosome, and binds to the minor groove of DNA in a sequence-specific manner. This mechanism is central to the efficacy of GO. It explains that cells expressing higher levels of CD33 are more susceptible to GO [38]. However, several patients with CD33-negative leukemia have also responded to GO [39]. Several studies have sought to explain the efficacy of GO on CD33-negative leukemia. One proposed explanation is that GO is partially moved into cell by CD33-independent endocytosis [39]. In their study, anti-CD33 blocking antibodies prevented the death of CD33-positive cells at low concentrations of GO, but not at higher concentrations. Another possible explanation is that CD33-negative leukemia cells may have a sub-threshold low amount of CD33, which reacts substantially with GO [33]. These ideas may also explain the mechanism of liver dysfunction, which has been observed in the GO treatment.
Calicheamicin
Calicheamicin, a hydrophobic enediyne antibiotic agent, was first isolated from the actinomycete Micromonospora echinospora ssp. Calichensis [7, 8]. It binds in a sequence-specific manner to the minor groove of DNA, and cleaves single and double-stranded DNAs by the removal of specific hydrogen atoms from the deoxyribose rings of DNAs [9]. DNA damage leads to apoptotic or non-apoptotic cell death due to mitochondrial damage [40–42]. We observed cell morphology after the incubation of GO by video-microscopy. Some cells exhibited apoptotic changes, while the remaining cells showed non-apoptotic features [43]. The cytotoxic mechanism of GO is the same as that of calicheamicin, except for the internalization via CD33. Cells incubated with calicheamicin undergo either temporary or permanent cell cycle arrest [43, 44]. In our study, transient G2/M arrest was observed prior to the increase of the hypodiploid portion in cell lines incubated with GO. Several molecular pathways, such as Chk1 and Chk2 phosphorylation and caspase 3, have been reported as playing roles in this process [45].
Drug resistance
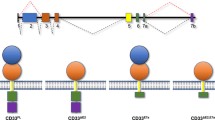
Multidrug resistant (MDR) is a phenomenon in which malignant cells develop cross-resistance to a variety of unrelated cytotoxic drugs. P-gp, a key player in MDR, is a membrane glycoprotein that actively pumps cytotoxic agents out from cells, and decreases intracellular drug accumulation [46, 47]. Calicheamicin derivatives, which are detached from GO in lysosomes, are also effluxed (Fig. 1) [48, 49]. GO showed less effect on sublines that expressed P-gp in vitro, even when they expressed sufficient levels of CD33 [43]. This phenomenon was confirmed by the combined use of GO and MDR modifiers, such as PSC833 and MS209, in the resistant sublines [43, 50, 51]. Cells that were persistently exposed to low-dose GO acquired resistance to GO and expressed P-gp [52]. These in vitro results were confirmed in phase I studies of GO. Good responders were more frequently observed in leukemia cases characterized by low dye efflux in vitro [33, 35]. In GO, MDR modifiers theoretically work only on intracellularly incorporated calicheamicin in CD33-positive cells. Our in vitro study suggested that the combination use of GO and MDR modifiers may be an ideal therapeutic approach for P-gp-expressing leukemia cells, assuming that the hematologic and non-hematologic toxicities are not worsened. Interestingly, similar results were obtained in studies using inotuzumab ozogamicin, a calicheamicin-conjugated anti-CD22 antibody, for lymphoid malignancies [53, 54].
Cyclosporin A (CyA), one of the well-known MDR modifiers, has in fact been administered as an adjunct to GO-containing chemotherapy in the treatment of AML [55–57]. CyA did not improve response rate or survival, although veno-occlusive disease (VOD) was observed in some patients [55]. The discrepancy between in vitro and in vivo effects may be explained by the possibility that CyA ablates the function of P-gp, which is widely distributed across critical organ systems, thus increasing adverse effects, and that the clinical outcome from the P-gp negative cases may thereby contribute to the non-significance of the results.
Several resistance mechanisms other than P-gp have also been suggested. Multidrug resistant-related protein 1 (MRP1), another transporter protein, is sometimes expressed in AML [58]. However, the clinical importance of MRP1 is relatively limited among the mechanisms of resistance to GO [59]. Other transporter proteins may have further limited effects.
The roles of bcl-2 and bcl-x, anti-apoptotic proteins, in the resistance to GO have been reported [60, 61]. The effect of GO was enhanced by bcl-2 antisense oligonucleotide, but reduced by overexpression of bcl-2 and bcl-x. Bax, Bak and stress-activated protein kinase may play a role in resistance to GO [62]. GO induced proapoptotic activation of Bak and Bax and stress-activated protein kinase in sensitive AML cells, but not in resistant ones. Peripheral benzodiazepine receptor ligand, PK11195, increased the sensitivity of AML cells to standard chemotherapeutics both by inhibiting P-gp and by promoting mitochondrial apoptosis. [61] It also increased sensitivity to GO in AML cells.
The activation of survival signaling pathways, such as PI3K/AKT, MEK/ERK and JAK/STAT, is reportedly associated with GO resistance in vitro in AML cells [63]. An AKT inhibitor, MK-2206, restored the resistance of GO and calicheamicin in resistant AML cells.
Delivery of GO to bone marrow may be important for enhancing the effect of GO [28, 38]. An excess of circulating CD33-positive cells decreased the effect of GO, and resulted in worse outcomes [27, 64]. However, high blast cell count is an adverse prognostic factor in leukemias treated with other anti-leukemic agents as well.
Several agents other than MDR modifiers reportedly increase the sensitivity of GO in vitro. G-CSF enhanced the effect of GO, and induced AML cells to enter G2/M and hypodiploid phase [65]. Valproic acid, a histone deacetylase inhibitor, can also strengthen the effect of GO [66]. However, the synergistic effect of GO with these agents has not been well elucidated in clinical studies.
Other resistance mechanisms have been suggested by several groups, including the alternative GO pharmacokinetics, and the reduction of CD33 on leukemia cells [36, 39, 43, 44]. In fact, multiple mechanisms may be at work in the development of resistance to GO.
GO monotherapy
In a phase I study conducted in the US, 40 patients with relapsed or refractory (relapsed/refractory) AML were treated by GO (0.25–9 mg/m2) [67]. Leukemia cells were eliminated from the blood and bone marrow of eight (20 %) of the 40 patients. Neutrophil counts recovered in five of these eight patients, but platelets recovered in only three. Patients who achieved complete remission (CR) without recovering platelet count more than 100 × 109/L were entered to the concept of CR with thrombocytopenia (CRp), which has been subsequently used in the evaluation of GO.
Phase II trials with GO were started at a dose of 9 mg/m2 (2-week intervals for two doses) [35]. A total of 142 patients with AML in first relapse were enrolled in the study. Of these patients, 30 % achieved overall response (OR), including CR and CRp. The median relapse-free survival (RFS) was 5.3 months. Grade 3 or 4 bilirubinemia was observed in 23 %, and hepatic transaminitis in 17 %. Veno-occlusive disease (VOD) was observed in seven patients (3 %), and three of these were fatal. Five patients, who received hematopoietic stem cell transplantation (HSCT) before the treatment of GO, did not have evident VOD. However, three of 27 patients who received HSCT after the treatment of GO died of VOD.
Based on these results, the Food and Drug Administration of US approved GO for relapsed CD33-positive AML in patients 60 years of age or older [68].
Larson et al. [69] treated 101 elderly patients with relapsed AML. The OR rate was 28 %, including CR 13 % and CRp 15 %. The OS was 5.4 months. Grade 3 or 4 bilirubinemia and transaminitis were observed in 24 and 15 %, respectively. Most of the liver dysfunctions were reversible.
Results for 128 patients with relapsed AML treated GO were compared with those for 128 patients treated with the combination chemotherapy with high-dose cytarabine (HiDAC) [70]. The OR rates in GO and HiDAC therapy were 38 and 41 %, respectively. GO treatment had a higher response rate if the previous remission duration was 3–10.5 months, whereas HiDAC treatment had a higher response rate if the duration of the previous remission was >19 months.
Twenty-four cases with relapsed/refractory AML were treated with GO (9 mg/m2 at 2-week intervals for two doses) [71]. The CR and the CRp rates were 13 and 8 %, respectively, and the median duration of second CR was 6 months. VOD was observed in one case.
Larson et al. [72] summarized three open-label, single-arm, phase II trials for AML in first recurrence. Patients received GO monotherapy (9 mg/m2 two doses separated by 2 weeks). CR or CRp was achieved in 13 % of cases in each study. The OR rates were not different between younger (28 %) and elderly (24 %) patients. The median OS and the RFS were 4.9 and 5.2 months, respectively. The median OS was >18.3, 16.5, 12.2, and 11.2 months for patients who received allogeneic HSCT, autologous HSCT, additional chemotherapy, or no additional therapy, respectively. Eight patients (17 %) developed VOD after HSCT. Five (19 %) of the 27 patients who underwent HSCT prior to GO developed VOD after treatment.
Fifty-seven patients with AML in first relapse received GO monotherapy (3 mg/m2 on days 1, 4, and 7) [73]. Fifteen patients (26 %) achieved CR and four (7 %) CRp. The median RFS was 11 months. Grade 3 or 4 liver toxicity and VOD were not observed.
The Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA)–the European Organization for Research on the Treatment of Cancer (EORTC) study assessed the efficacy of GO monotherapy (9 mg/m2 on days 1 and 15) in 40 elderly patients with AML, who were not considered eligible for conventional chemotherapy due to advanced age or poor performance status [74]. The OR rate was 17 %, which was poorer in elderly patients. The median OS was 4.3 months, and the 1-year OS rates were 34 %. Grade 3 or 4 liver toxicity was observed in 10 %.
In Japan, 20 patients with relapsed/refractory AML received GO (9 mg/m2 for two doses at 2-week intervals) [75]. CR and CRp were achieved in 5 (25 %) and 1 (5 %) patients, respectively. The median OS was 420 days. Grade 3 or 4 transaminitis was observed in 1 patient, but VOD was not.
The results from the study containing a relatively large number of patients are summarized in Table 1. These results encouraged the initiation of trials of combination chemotherapy including GO.
Post-marketing study of GO monotherapy
The efficacy of GO on relapsed/refractory AML, including acute promyelocytic leukemia (APL), in a post-marketing surveillance study of GO was reported in Japan (http://pfizerpro.jp/). A total of 760 patients with relapsed/refractory AML were enrolled, of which 503 were evaluable. In AML, CR and CRp were achieved in 10 and 8 %, respectively; while in APL 48 and 9 %, respectively (Table 5). In AML, the OS and the RFS at 2 years were 14 and 20 %, respectively; while these were 63 and 71 %, respectively, in APL. Treatment-related adverse events of GO (grade 3 or 4) in AML patients were infusion reaction (22 %), infection (27 %), bleeding (9 %), lung damage (3 %) and reversible VOD (4 %) [76]. The incidence of VOD in patients administered GO after HSCT was 5.9 %, which showed no change compared to those without HSCT.
Combination chemotherapy with GO for relapsed or/and refractory AML
Based on the data of GO monotherapy for relapsed/refractory AML, many combination chemotherapies with GO have been conducted.
A pilot study of GO combined with topotecan and Ara-C (MTA) was assessed in patients with refractory AML [77]. MTA consisted of GO (9 mg/m2 on day 1), AraC (1 g/m2 on days 1–5) and topotecan (1.25 mg/m2 on days 1–5). A group of 17 patients with relapsed/resistant AML or advanced MDS received 20 courses of MTA. CR was achieved in 12 %. The median OR was 8.2 weeks. Five patients (29 %) developed grade 3 or 4 hepatic transaminitis; one of them died due to VOD.
GO (6 mg/m2 on days 1 and 15) was administered with idarubicin (IDA) (12 mg/m2 on days 2–4) and Ara-C (1.5 g/m2 on days 2–5) (MIA) [78]. Fourteen patients with relapsed/refractory AML were treated with MIA. CR and CRp were achieved in 21 % of each group. The median OS was 8 weeks, and the median failure-free survival of CR patients was 27 weeks. Grade 3 or 4 hepatic transaminitis was observed in 57 %, and VOD was in 14 %.
The MDAC regimen, consisted of GO (6 mg/m2 on day 6), Ara-C (1 g/m2 on days 1–5), liposome-encapsulated DNR (75 mg/m2 on days 6–8) and CyA (on day 6) (MDAC), was conducted in 11 patients with relapsed/refractory AML [55]. CR and CRp were achieved in one case each, respectively. Grade 3 or 4 bilirubinemia was observed in 54 %, and transaminitis in 9 %.
The MFAC regimen, consisted of GO (4.5 mg/m2 on day 1), CyA (6 mg/kg on day 1), fludarabine (15 mg/m2 every 12 h on days 2–4) and Ara-C (0.5 g/m2 every 12 h on days 2–4), was conducted in 32 patients with relapsed/refractory AML [56]. CR and CRp were achieved in 28 and 6 %, respectively. The median OS was 5.3 months, and the 1-year OS rate was 19 %. Grade 3 or 4 hyperbilirubinemia was observed in 44 %, transaminitis in 18 %, and VOD in 9 %.
Twenty-two patients in CR commenced IDA and AraC (IA) alternating with MFAC or vice versa for 9 months from the date of CR [57]. The failure-free and the 1-year OS rates were 32 and 55 %, respectively. Grade 3 or 4 toxicities were not different between the MFAC and IA regimen.
Nine elderly patients with AML (five untreated and four relapsed/refractory) were treated with GO (6 mg/m2 on day 1, 4 mg/m2 on day 8) in combination with AraC (100 mg/m2 as continuous infusion on days 1–7) [79]. CR was achieved in five patients. The median CR duration was 10 months, and the median OS was 6 months. Grade 3 or 4 bleeding was observed in 44 %. VOD was not observed.
MIDAM regimen, consist of GO (9 mg/m2 on day 1), AraC (1 g/m2 every 12 h on days 1–5), mitoxantrone (MIT, 12 mg/m2 on days 1–3), was conducted in 17 patients with refractory/relapsed AML [80]. CR and CRp were achieved in 70 and 6 %, respectively. The median OS and RFS were 11 months each. Probability of the 1-year OS and RFS was 48 and 36 %, respectively. VOD was observed in one patient (6 %).
The Cancer and Leukemia Group B (CALGB) treated patients with relapsed/refractory AML with HiDAC (3 g/m2 for 5 days) associated with GO (9 mg/m2) [81]. HiDAC plus GO 9 mg/m2 on day 7 and 4.5 mg/m2 on day 14 was not tolerated, but HiDAC followed by GO 9 mg/m2 on day 7 was safe. CR was achieved in 32 %. The median OS was 8.9 months. Serious VOD was not observed. They concluded that the regimen merits further study for use both in remission induction and consolidation therapies.
Oblimersen, Bcl-2 antisense, has been shown to enhance the apoptotic activity of various antileukemic agents. Oblimersen (7 mg/kg, days 1–7 and 15–21) was administered with GO (9 mg/m2 on days 4 and 18) in 48 elderly patients with relapsed AML [60]. Twelve patients (25 %) achieved OR. The median OS for all patients enrolled was 2.3 months. Grade 3 and grade 4 toxicities were sepsis (12 %) urinary tract infection (8 %), pneumonia (6 %) and respiratory events (31 %).
In these studies, GO was used in the dosage from 4.5 to 9 mg/m2 with other chemotherapeutic agents. (Table 2) Although the outcomes of these studies were not sufficient to demonstrate efficacy overall, usefulness was expected in de novo AML.
Phase II trials of combination chemotherapy with GO for de novo AML and MDS
Based on the results from relapsed/refractory AML, many trials of combination chemotherapy with GO for de novo AML have been conducted. Results from several relatively large groups of patients are shown in Table 3.
Fifty-one patients aged 65 years or older with de novo AML, refectory anemia (RA) with excess of blasts in transformation (RAEBT) or RA with excess blasts were treated with GO (RAEB) [82]. GO was given in doses of 9 mg/m2 on days 1 and 8, or on days 1 and 15, with or without IL-11 (15 μg/kg on days 3–28). CR was achieved in 8 % in the GO without IL-11 group, and 36 % in the GO with IL-11 group. However, the CR rate and OS were inferior in patients treated with GO compared to historical data from those treated with IDA plus AraC.
Fifty-nine newly diagnosed patients (39 patients with AML, and 20 patients with RAEB/RAEBT) were treated with the MFAC regimen including GO (6 mg/m2 on day 1); fludarabine (15 mg/m2 on days 2–6), AraC (0.5 g/m2 on days 2–6) and cyclosporine A (6 mg/kg on days 1 and 2) [56]. CR and CRp were achieved in 46 and 2 %, respectively. The median OS was 8 months. The 1-year OS and EFS were 38 and 27 %, respectively. Grade 3 or 4 toxicity was observed, including bilirubinemia in 31 % and transaminitis in 7 % of the patients. Four patients (7 %) developed VOD.
The preliminary efficacy of GO with intensive chemotherapy was analyzed in 72 patients with younger de novo AML, aged 17–59 years [Medical Research Council (MRC) AML15 trial] [65]. Sixty-four patients received induction chemotherapy, such as DAT (DNR, AraC, thioguanine), DA (DNR and AraC) or FLAG-Ida (fludarabine, AraC, G-CSF, IDA) with GO (3 mg or 6 mg/m2 on day 1). GO 3 mg/m2 in the first course was feasible. However, neither GO 6 mg/m2 in the first course nor GO 3 mg/m2 in consecutive courses was feasible. VOD was frequently observed in the treatment including thioguanine. OR was achieved in 86 %. These results provided useful data for the phase III trial conducted by the same group.
Fifty-three patients with younger de novo AML were treated with DNR (45 mg/m2 on days 1–3), AraC (100 mg/m2 on days 1–7) and GO (6 mg/m2 on day 4) [83]. OR was achieved in 83 % of the patients. Four of eight patients who underwent HSCT < 115 days from the first day of induction developed VOD; while none of 12 patients who underwent HSCT > 115 days developed VOD. These data have been included in the product information. HSCT before and after the administration of GO should thus be conducted with vigilance toward symptoms of VOD. In the study reported by the same group, 21 elder patients with de novo AML were treated with AraC (100 mg/m2 on days 1–7) and GO (6 mg/m2 on day 1 and 8). OR was achieved in 43 %. Grade 3 or 4 bilirubinemia was observed in 6 %, but VOD was not.
In the GIMEMA-EORTC study, GO (9 mg/m2 on days 1 and 15) followed by conventional chemotherapy consisting of MIT, AraC, and ETP (MICE) was administered in 57 elderly patients with de novo AML [84]. OR was achieved in 54 %, with CR in 35 % and CRp in 19 %. One-year OS was 34 %. VOD developed in 9 %.
Combination therapy including G-CSF, AraC, and GO (G-AraMy) was administered in 53 elderly patients with untreated or primary refractory/relapsed AML [85]. Of these, 27 received G-AraMy1 and 26 G-AraMy2 protocols. G-AraMy included G-CSF (5 μg/kg on days 1–8), AraC (100 mg/m2 continuously on days 4–8 in G-AraMy1 or days 2–8 in G-AraMy2) and GO (6 mg/m2 on day 9). The outcomes were not different between the two groups. VOD was observed in 2 %. The response rate and toxicity profile were not different between untreated and primary resistant/relapsed AML, or between de novo and secondary AML.
The studies, reported by Kell et al. and De Angelo et al., [65, 83] focused on younger patients with AML. Considerably positive outcomes were obtained in these two studies and encouraged the group to plan open label phase III trials. Outcomes from elderly patients with AML or MDS in contrast were unsatisfactory.
Phase III study with GO
Based on the result of phase I and II trials, the efficacy of GO has been studied in an open label phase III trial (Table 4).
The Southwest Oncology Group (SWOG) study-S0106 reported the benefit and toxicity of adding GO to standard therapy in 627 patients with de novo AML [86]. Patients were randomized to receive induction therapy with DNR (45 mg/m2 on days 1–3) and AraC (100 mg/m2 on days 1–7) and GO (6 mg/m2 on day 4) (AD + GO) or standard induction therapy with DNR (60 mg/m2 on days 1–3) and AraC (100 mg/m2 on days 1–7) (AD). Patients achieving CR received consolidation therapy with three courses of HiDAC. Patients in remission were re-randomized to the treatment of GO (5 mg/m2 every 28 days, three doses) or observation. The OR rate was 74 % in both induction arms. The RFS was not significantly different between two arms. Fatal adverse events were significantly increased in the AD + GO arm. Clinical outcomes were not improved by GO, and a higher fatality rate was observed on addition of GO.
The results of SWOG-S0106 triggered Pfizer Corp. to voluntarily withdraw GO from the market in 2010. However, several problems in the study have been raised. The doses of DNR were different between the two arms, making it difficult to precisely determine any additional efficacy of GO. Additionally, the induction mortality in the control arm was extremely low compared to other studies. The mortality rate (5.8 %) in GO arm may be within the acceptable range in other studies. Other studies have sought to address these problems and resolve the additional efficacy of GO.
In a subsequent study, 238 patients with de novo AML and intermediate karyotype were treated with standard chemotherapy with or without GO [87]. GO (6 mg/m2) was added to standard 3 + 7 induction, and to a consolidation of MIT and AraC. The CR rate and early death rate were unchanged in both groups. Grade 3 or 4 hepatic toxicities were increased in GO arm. The EFS and the OS were not changed in both treatment arms. In patients who did not receive HSCT, EFS was significantly higher in the GO arm (54 % vs 27 %) while OS did not improve.
In the MRC-AML15 trial, 1,113 patients with de novo AML, excluding APL, were randomly assigned to receive either of the following three induction treatments: DNR and AraC; DNR, ETP and AraC; or fludarabine, IDA, AraC and G-CSF; with or without GO (3 mg/m2) [88]. In remission, 948 patients were randomly assigned to GO (3 mg/m2) in combination with amsacrine, AraC and ETP or HiDAC (1.5 g or 3 g/m2). The CR rate or the OS was not significantly different. Survival benefit of GO was observed in patients with favorable cytogenetics, but not in patients with high-risk disease. GO did not increase toxicity. This study showed that GO can improve survival for patients with favorable risk AML.
In other results from UK and Denmark, 1,115 patients with AML or high-risk MDS were randomly assigned to receive induction chemotherapy with either DNR (50 mg/m2 on days 1, 3, 5) and Ara-C (100 mg/m2 twice a day on days 1–10) or DNR and clofarabine (20 mg/m2 on days 1–5), with or without GO (3 mg/m2) [89]. No difference in OR rate was observed between the two arms. GO did not increase toxicity and mortality. Three-year cumulative incidence of relapse was significantly lower, and 3-year OS was significantly better in the patients treated with GO.
Two hundred seventy-eight elderly patients with de novo AML were received DNR (60 mg/m2 on days 1–3) and AraC (200 mg/m2 for 7 days) without (control group) or with GO (3 mg/m2 on days 1, 4, and 7) [90]. The OR rate was not different between the two groups. The 2-year EFS, OS, and RFS were significantly improved by the addition of GO. GO did not increase the risk of death from toxicity. They concluded that the fractionated lower doses of GO were safe and improved outcomes.
These recent results demonstrated some advantage for patients treated with GO. In addition, induction mortality was not increased in these studies. Efficacy was observed, typically in patients with favorable risk, and sometimes in intermediate risk. The reason for this has not been elucidated. Molecular analyses concomitant with clinical outcome data will be needed to address this in the future.
Doses of GO and anthracyclines differed in these studies. From the recent studies, 3–6 mg/m2 of GO for 1 day and 50–60 mg/m2 of DNR for 3 days may be chosen. Ongoing and future studies may help to demonstrate the optimal dosage of these agents in combination chemotherapy including GO.
Low-dose AraC (LDAC, 20 mg, twice a day) was administered randomly with or without GO (5 mg) in 495 elderly patients with AML [91]. GO improved OR rate, but not OS, at 1 year. Improvement of OS in elderly AML remains a challenge for treatments, including GO.
GO for pediatric AML
Reports of results from pediatric patients treated with GO remain relatively limited. Twelve children with relapsed/refractory AML received GO (1.8–9 mg/m2 at 2-week intervals for 1–2 doses) [92]. Five children responded to treatment with blast reduction, but no child achieved CR. One patient treated GO after HSCT developed reversible VOD.
Seventeen children with relapsed/refractory AML received GO (3 mg/m2 on days 1, 4 and 7) plus AraC (100 mg/m2 for 7 days) (GOCYT), and seven of these received GO-based consolidation [93]. The OR rate was 35 and 53 % after induction therapy and consolidation therapy with GO, respectively. VOD was not observed.
Thirty children with AML, who were refractory to re-induction at first relapse, received GO (7.5 mg/m2 at 2-week intervals for 1–2 doses) [94]. The OR rate was 37 %, and 3-year OS was 27 %. Grade 3–4 bilirubinemia and transient transaminitis were observed in one and two cases, respectively.
Twenty-nine children with relapsed/refractory AML received GO (6, 7.5 or 9 mg/m2 at 2-week intervals for two doses) [95]. In 13 (45 %) of them, GO was administered after HSCT. The OR rate was 97 %. Grade 3 or 4 bilirubinemia and transaminitis were observed in 7 and 21 %, respectively. VOD was observed in seven (26 %) patients, six of whom had received HSCT.
A group of 230 children with untreated AML were treated with high-dose (18 g/m2) or low-dose (2 g/m2) AraC, DNR and ETP (ADE; induction 1), followed by ADE with or without GO (ADE; induction 2) [96]. ADE plus GO was shown to be feasible. CR was achieved in 80 % after induction 1, and 94 % after induction 2. The 3-year EFS and OS were 63 and 71 %, respectively.
In the Children’s Oncology Group (COG)-AAML00P2 trial, the maximum tolerated dose (MTD) of GO in combination with AraC and MIT, and with AraC and l-asparaginase was analyzed, and was concluded to be 3 and 2 mg/m2, respectively [97].
A group of 350 children with previously untreated AML were enrolled to the COG-AAML03P1 [98]. GO (3 mg/m2 on day 6 of the first course) was administrated with chemotherapy with DNR, AraC, and ETP. The CR rate was 83 % after the first course. The mortality rate after the first course was 1.5 %. The 3-year EFS and OS rates were 53 and 66 %, respectively.
The Nordic Society of Pediatric Hematology and Oncology (NOPHO)-AML 2004 trial estimated post-consolidation effect of GO (6 mg/m2 at 3-week intervals for two doses) in children with AML. Of a total of 120 patients randomized, 59 received GO [99]. GO was well tolerated, but the relapse rate, median time to relapse, the 5-year EFS and OS were not different between the two groups.
Given these equivocal results, the efficacy of GO in children with AML remains uncertain.
In vitro efficacy of GO for APL
In our in vitro study, GO showed equivalent effects on ATRA and/or ATO-resistant APL cells unless they expressed P-gp [100]. The cell lines used in our study are NB4, ATRA-resistant NB4 (NB4/RA), P-gp-positive NB4 and NB4/RA (NB4/MDR and NB4/RA/MDR) and ATO-resistant NB4 (NB4/As). GO did not exhibit cross-resistance with ATRA- and ATO-resistance. GO is likely useful for induction therapy after resistance to these drugs has been acquired.
Relapsed and recurrent APL
While ATRA combined with chemotherapy has been the standard treatment for patients with APL, approximately 20 % undergo relapse [101–103]. Several salvage therapies, including Am80, ATO, and stem cell transplantation, have been introduced for the treatment of APL [104, 105]. GO was also administered to APL, and the successful use of this therapy has been reported for patients with newly diagnosed or relapsed APL [106–108].
Several ideas have been proposed to explain the efficacy of GO for APL [100, 109]. First, a large amount of CD33 is commonly expressed on the surface of APL cells. Second, the level of P-gp on the surface of APL cells is lower than that of AML. Third, APL cells are highly sensitive for free calicheamicin.
Several investigators have reported the clinical efficacy of GO for patients of relapsed APL (Table 5). Lo-Coco et al. [107] reported that 14 of 16 patients with molecularly relapsed APL achieved molecular remission (MR) after GO monotherapy (6 mg/m2 at 2-week intervals for three doses). Of 14 responders, seven (50 %) remained in sustained MR for a median of 15 months. GO was administered again in two patients with relapse, and both obtained a new MR.
We treated patients, who were in a third morphologic relapse with a considerable number of APL cells, by GO monotherapy (9 mg/m2 on days 1 and 15). They developed prominent DIC after GO treatment [108]. Both patients achieved CR. One of the patients was treated with consolidation chemotherapy, but the other was not. Both patients had a considerably long remission period. GO may represent another treatment option if stem cell transplantation is not being considered in the near future. Treatment with GO may transiently increase the severity of DIC, as APL cells collapse rapidly.
Aribi et al. [109] reported the efficacy of a combination therapy consisting of ATO, ATRA, and GO in eight patients with APL in first recurrence. Patients were treated with ATO until CR, and then received the consolidation therapy including ATO, ATRA and GO (9 mg/m2) once a month for 10 months. The second CR was longer than the first CR in 75 %. All patients achieved MR. There were no grade 3 or 4 non-hematological toxicities.
GO (3–6 mg/m2 for two doses) was administered in three elderly patients with APL who had molecular relapse and were deemed unfit for intensive chemotherapy [110]. All of the patients achieved second MR, and did not relapse. The study suggested that low-dose GO is effective to treat MR in elderly APL patients.
In a Japanese post-marketing study of GO for APL, whose results were partially described in the previous section [76], remission duration of first CR and number of relapses influenced CR rates, but previous usage of ATO did not. Treatment-related adverse events (grade 3 or 4) in APL were similar to those seen in AML. GO may be more effective in APL compared to AML, and relatively safe in relapsed/refractory APL.
These reports show that GO is effective for APL patients with molecularly relapsed and advanced relapsed forms of the disease. These data also support the use of GO treatment for newly diagnosed APL.
Newly diagnosed APL
In the US study, 19 newly diagnosed patients with APL were treated with GO (9 mg/m2) [106]. Patients received eight additional courses (once every 4–5 weeks) of GO and ATRA after CR was achieved. GO was shown to be feasible. CR and MR were achieved in 84 and 74 %, respectively. All patients tested during the study were PCR-negative 2–4 months from CR.
In another study, 19 untreated patients with high-risk APL were treated with GO (9 mg/m2 on day 1) in addition to ATRA plus ATO as induction therapy [111]. Fifteen (79 %) out of 19 cases achieved CR, three cases relapsed. The authors suggested that GO was still effective for high-risk APL treated with ATRA and ATO.
In a third study, 82 newly diagnosed APL patients were treated with ATRA, ATO and GO [112]. The first cohort included 65 patients, who received ATRA followed by ATO. GO (9 mg/m2 on day 1) was administered in high risk. The second cohort included 17 patients, who received ATRA and ATO simultaneously. GO (9 mg/m2) was added on high risk or increase of WBC count above 30 × 109/L during induction. CR was achieved in 95 and 81 % cases with low-risk and high-risk APL, respectively; 3-year OS was 85 %. The addition of GO to ATRA plus ATO may represent a promising initial therapy for high-risk APL.
Larger clinical studies of GO for the treatment of relapsed/refractory APL are warranted to obtain clearer clinical evidence. The results may suggest how GO will be integrated into the management of APL. The Japan Adult Leukemia Study Group (JALSG) has also launched prospective studies for APL that include GO.
GO has introduced a new perspective into the treatment of AML. However, the second evaluation of this treatment did not yield positive results. Recent studies have shown the efficacy of GO in AML, with favorable risk in APL as well. Subsequent evaluations should focus on the efficacy of GO in core binding factor (CBF) leukemia and its mechanism of action, which may lead to the re-approval of GO.
References
Tallman MS, Gillands DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–63.
Miyawaki S. Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol. 2012;96:171–7.
Stein EM, Tallman MS. Remission induction in acute myeloid leukemia. Int J Hematol. 2012;96:164–70.
Kühnl A, Grimwade D. Molecular markers in acute myeloid leukaemia. Int J Hematol. 2012;96:153–63.
Naoe T, Kiyoi H. Gene mutations of acute myeloid leukemia in the genome era. Int J Hematol. 2013;97:165–74.
Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadesin family of cellular interaction molecules. Blood. 1995;85:2005–12.
Lee MD, Dunne TS, Siegel MM, Chang CC, Morton GO, Borders DB. Calicheamicins, a novel family of antitumor antibiotics. 1: chemistry and partial structure of calicheamicin γ I1 . J Am Chem Soc. 1987;109:3464–6.
Lee MD, Dunne TS, Chang CC, Ellestad GA, Siegel MM, Morton GO, McGahren WJ, Borders DB. Calicheamicins, a novel family of antitumor antibiotics. 2: chemistry and structure of calicheamicin γ I1 . J Am Chem Soc. 1987;109:3466–8.
Zein N, Poncin M, Nilakantan R, Ellestad GA. Calicheamicin γ I1 and DNA: molecular recognition process responsible for site-specificity. Science. 1989;244:697–9.
Hangeland JJ, De Voss JJ, Heath JA, Townsend CA. Specific abstraction of the 5′(S)- and 4′-deoxyribosyl hydrogen atoms from DNA by calicheamicin γ I1 . J Am Chem Soc. 1992;114:9200–2.
Tchilian EZ, Beverley PC, Young BD, Watt SM. Molecular cloning of two isoforms of the murine homolog of the myeloid CD33 antigen. Blood. 1994;83:3188–98.
Gao Z, McAlister VC, Williams GM. Repopulation of liver endothelium by bone-marrow-derived cells. Lancet. 2001;357:932–3.
Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell–cell interactions and signaling. Curr Opin Struct Biol. 2002;12:609–15.
Taylor VC, Buckley CD, Douglas M, Cody AJ, Simmons DL, Freeman SD. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. Biol Chem. 1999;274:11505–12.
Andrews RG, Torok-Storb B, Bernstein ID. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983;62:124–32.
Andrews RG, Takahashi M, Segal GM, Powell JS, Bernstein ID, Singer JW. The L4F3 antigen is expressed by unipotent and multipotent colony-forming cells but not by their precursors. Blood. 1986;68:1030–5.
Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8:521–34.
Robertson MJ, Soiffer RJ, Freedman AS, Rabinowe SL, Anderson KC, Ervin TJ, et al. Human bone marrow depleted of CD33-positive cells mediates delayed but durable reconstitution of hematopoiesis: clinical trial of MY9 monoclonal antibody-purged autografts for the treatment of acute myeloid leukemia. Blood. 1992;79:2229–36.
Wagner JE, Collins D, Fuller S, Schain LR, Berson AE, Almici C, et al. Isolation of small, primitive human hematopoietic stem cells: distribution of cell surface cytokine receptors and growth in SCID-Hu mice. Blood. 1995;86:512–23.
Dinndorf PA, Andrews RG, Benjamin D, Ridgway D, Wolff L, Bernstein ID. Expression of normal myeloid-associated antigens by acute leukemia cells. Blood. 1986;67:1048–53.
Scheinberg DA, Tanimoto M, McKenzie S, Strife A, Old LJ, Clarkson BD. Monoclonal antibody M195: a diagnostic marker for acute myelogenous leukemia. Leukemia. 1989;3:440–5.
Terstappen LW, Safford M, Konemann S, Loken MR, Zurlutter K, Buchner T, et al. Flow cytometric characterization of acute myeloid leukemia. Part II. Phenotypic heterogeneity at diagnosis. Leukemia. 1992;6:70–80.
Putti MC, Rondelli R, Cocito MG, Aricó M, Sainati L, Conter V, et al. Expression of myeloid markers lacks prognostic impact in children treated for acute lymphoblastic leukemia: Italian experience in AIEOP-ALL 88–91 studies. Blood. 1998;92:795–801.
Jilani I, Estey E, Huh Y, Joe Y, Manshouri T, Yared M, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Path. 2002;118:560–6.
Iwamoto S, Deguchi T, Ohta H, Kiyokawa N, Tsurusawa M, Yamada T, et al. Flow cytometric analysis of de novo acute lymphoblastic leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2011;94:185–92.
Hernández-Caselles T, Martínez-Esparza M, Pérez-Oliva AB, Quintanilla-Cecconi AM, García-Alonso A, Alvarez-López DM, García-Peñarrubia P. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J Leukoc Biol. 2006;79:46–58.
Sheinberg DA, Lovett D, Divgi CR, Graham MC, Berman E, Pentlow K, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J Clin Oncol. 1991;9:478–90.
Caron PC, Co MS, Bull MK, Avdalovic NM, Queen C, Scheinberg DA. Biological and immunological features of Humanized M195 (Anti-CD33) monoclonal antibodies. Cancer Res. 1992;52:6761–7.
Borthakur G, Rosenblum MG, Talpaz M, Daver N, Ravandi F, Faderl S, et al. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica. 2013;98:217–21.
La Russa VF, Griffin JD, Kessler SW, Cutting MA, Knight RD, Blattler WA, et al. Effects of anti-CD33 blocked ricin immunotoxin on the capacity of CD34+ human marrow cells to establish in vitro hematopoiesis in long-term marrow cultures. Exp Hematol. 1992;20:442–8.
Appelbaum FR, Matthews DC, Eary JF, Badger CC, Kellogg M, Press OW, et al. The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation. 1992;54:829–33.
van der Velden VHJ, Boeckx N, Jedema I, te Marvelde JG, Hoogeveen PG, Boogaerts M, et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia. 2004;18:983–8.
Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19:176–82.
Matsui H, Takeshita A, Naito K, Shinjo K, Shigeno K, Maekawa M, et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia. 2002;16:813–9.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, Mylotarg Study Group, et al. Efficacy and safety of gemutuzumab ozogamicin in patients with CD33-positive acute myloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–54.
Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13:47–58.
McGrath MS, Rosenblum MG, Philips MR, Scheinberg DA. Immunotoxin resistance in multidrug resistant cells. Cancer Res. 2003;63:72–9.
Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105:1295–302.
Jedema I, Barge RM, van der Velden VH, Nijmeijer BA, van Dongen JJ, Willemze R, et al. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia. 2004;18:316–25.
Zhao B, Konno S, Wu JM, Oronsky AL. Modulation of nicotinamide adenine dinucleotide and poly(adenosine diphosphoribose) metabolism by calicheamicin gamma 1 in human HL-60 cells. Cancer Lett. 1990;50:141–7.
Nicolaou KC, Pitsinos EN, Theodorakis EA, Saimoto H, Wrasidlo W. Synthetic calicheamicin mimics with novel initiation mechanisms: DNA cleavage, cytotoxicity, and apoptosis. Chem Biol. 1994;1:57–66.
Lode HN, Reisfeld RA, Handgretinger R, Nicolaou KC, Gaedicke G, Wrasidlo W. Targeted therapy with a novel enediyene antibiotic calicheamicin ϑ1 effectively suppresses growth and dissemination of liver metastases in a syngeneic model of murine neuroblastoma. Cancer Res. 1998;58:2925–8.
Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, Shinjo K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14:1436–43.
Matsui H, Takeshita A, Naito K, Shinjo K, Shigeno K, Maekawa M, et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia. 2002;16:810–9.
Amico D, Barbui AM, Erba E, Rambaldi A, Introna M, Golay J. Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: role of Chk1 and Chk2 phosphorylation and caspase 3. Blood. 2003;101:4589–97.
Kartner N, Evernden-Porelle D, Bradley G, Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature. 1985;316:820–3.
Gottesman MM, Pastan I, Ambudkar SV. P-glycoprotein and multidrug resistance. Curr Opin Genet Dev. 1996;6:610–7.
Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2004;102:1466–73.
Linenberger ML, Hong T, Flowers D, Sievers EL, Gooley TA, Bennett JM, et al. Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood. 2001;98:988–94.
Baba M, Nakanishi O, Sato W, Saito A, Miyama Y, Yano O, et al. Relationship between multidrug resistant gene expression and multidrug resistant-reversing effect of MS-209 in various tumor cells. Cancer Chemother Pharmacol. 1995;36:361–7.
Friche E, Jensen PB, Nissen NI. Comparison of cyclosporin A and SDZ PSC833 as multidrug-resistance modulators in a daunorubicin-resistant Ehrlich ascites tumor. Cancer Chemother Pharmacol. 1992;30:235–7.
Matsumoto T, Jimi S, Hara S, Takamatsu Y, Suzumiya J, Tamura K. Importance of inducible multidrug resistance 1 expression in HL-60 cells resistant to gemtuzumab ozogamicin. Leuk Lymphoma. 2012;53:1399–405.
Takeshita A, Yamakage N, Shinjo K, Ono T, Hirano I, Nakamura S, et al. CMC-544 (inotuzumab ozogamicin), an anti-CD22 immuno-conjugate of calicheamicin, alters the levels of target molecules of malignant B-cells. Leukemia. 2009;23:1329–36.
Takeshita A, Shinjo K, Yamakage N, Ono T, Hirano I, Matsui H, et al. CMC-544 (inotuzumab ozogamicin) shows less effect on multidrug resistant cells: analyses in cell lines and cells from patients with B-cell chronic lymphocytic leukaemia and lymphoma. Br J Haematol. 2009;146:34–43.
Apostolidou E, Cortes J, Tsimberidou A, Estey E, Kantarjian H, Giles FJ. Pilot study of gemtuzumab ozogamicin, liposomal daunorubicin, cytarabine and cyclosporine regimen in patients with refractory acute myelogenous leukemia. Leuk Res. 2003;27:887–91.
Tsimberidou A, Estey E, Cortes J, Thomas D, Faderl S, Verstovsek S, et al. Gemtuzumab, fludarabine, cytarabine, and cyclosporine in patients with newly diagnosed acute myelogenous leukemia or high-risk myelodysplastic syndromes. Cancer. 2003;97:1481–7.
Tsimberidou A, Cortes J, Thomas D, Garcia-Manero G, Verstovsek S, Faderl S, et al. Gemtuzumab ozogamicin, fludarabine, cytarabine and cyclosporine combination regimen in patients with CD33 + primary resistant or relapsed acute myeloid leukemia. Leuk Res. 2003;27:893–7.
Legrand O, Zittoun R, Marie J-P. Role of MRP1 in multidrug resistance in acute myeloid leukemia. Leukemia. 1999;13:578–84.
Cianfriglia M, Mallano A, Ascione A, Dupuis ML. Multidrug transporter proteins and cellular factors involved in free and mAb linked calicheamicin-gamma1 (gentuzumab ozogamicin, GO) resistance and in the selection of GO resistant variants of the HL60 AML cell line. Int J Oncol. 2010;36:1513–20.
Moore J, Seiter K, Kolitz J, Stock W, Giles F, Kalaycio M, et al. A Phase II study of Bcl-2 antisense (oblimersen sodium) combined with gemtuzumab ozogamicin in older patients with acute myeloid leukemia in first relapse. Leuk Res. 2006;30:777–83.
Walter RB, Raden BW, Cronk MR, Bernstein ID, Appelbaum FR, Banker DE. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004;103:4276–84.
Haag P, Viktorsson K, Lindberg ML, Kanter L, Lewensohn R, Stenke L. Deficient activation of Bak and Bax confers resistance to gemtuzumab ozogamicin-induced apoptotic cell death in AML. Exp Hematol. 2009;37:755–66.
Rosen DB, Harrington KH, Cordeiro JA, Leung LY, Putta S, Lacayo N, et al. AKT signaling as a novel factor associated with in vitro resistance of human AML to gemtuzumab ozogamicin. PLoS ONE. 2013;8:e53518. doi:10.1371.
van der Velden VH, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS, van Dongen JJ. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97:3197–204.
Kell WJ, Burnett AK, Chopra R, Yin JA, Clark RE, Rohatiner A, et al. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood. 2003;102:4277–83.
ten Cate B, Samplonius DF, Bijma T, de Leij LF, Helfrich W, Bremer E. The histone deacetylase inhibitor valproic acid potently augments gemtuzumab ozogamicin-induced apoptosis in acute myeloid leukemic cells. Leukemia. 2007;21:248–52.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Löwenberg B, Dombret H, et al. Mylotarg Study Group. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–54.
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–6.
Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, Mylotarg Study Group, et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia. 2002;16:1627–36.
Leopold LH, Berger MS, Cheng SC, Cortes-Franco JE, Giles FJ, Estey EH, et al. Comparative efficacy and safety of gemtuzumab ozogamicin monotherapy and high-dose cytarabine combination therapy in patients with acute myeloid leukemia in first relapse. Clin Adv Hematol Oncol. 2003;1:220–5.
Piccaluga PP, Martinelli G, Rondoni M, Malagola M, Gaitani S, Isidori A, et al. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma 2004;45:1971–5.
Larson RA, Sievers EL, Stadtmauer EA, Löwenberg B, Estey EH, Dombret H, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–52.
Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia. 2007;21:66–71.
Amadori S, Suciu S, Stasi R, Willemze R, Mandelli F, Selleslag D, et al. Gemtuzumab ozogamicin (Mylotarg) as single-agent treatment for frail patients 61 years of age and older with acute myeloid leukemia: final results of AML-15B, a phase 2 study of the European Organisation for Research and Treatment of Cancer and Gruppo Italiano Malattie Ematologiche dell’Adulto Leukemia Groups. Leukemia. 2005;19:1768–73.
Kobayashi Y, Tobinai K, Takeshita A, Naito K, Asai O, Dobashi N, et al. Phase I/II study of humanized anti-CD33 antibody conjugated with calicheamicin, gemtuzumab ozogamicin, in relapsed or refractory acute myeloid leukemia: final results of Japanese multicenter cooperative study. Int J Hematol. 2009;89:460–9.
Takeshita A, Ono T, Kojima Y, Kyo T, Asou N, Suzushima H, et al. Efficacy of gemtuzumab ozogamicin (GO) monotherapy on relapsed/refractory acute promyelocytic leukemia (APL). Blood. 2011;118:Abst. #1532.
Cortes J, Tsimberidou AM, Alvarez R, Thomas D, Beran M, Kantarjian H, et al. Mylotarg combined with topotecan and cytarabine in patients with refractory acute myelogenous leukemia. Cancer Chemother Pharmacol. 2002;50:497–500.
Alvarado Y, Tsimberidou A, Kantarjian H, Cortes J, Garcia-Manero G, Faderl S, et al. Pilot study of Mylotarg, idarubicin and cytarabine combination regimen in patients with primary resistant or relapsed acute myeloid leukemia. Cancer Chemother Pharmacol. 2003;51:87–90.
Piccaluga PP, Martinelli G, Rondoni M, Malagola M, Gaitani S, Visani G, Baccarani M. First experience with gemtuzumab ozogamicin plus cytarabine as continuous infusion for elderly acute myeloid leukaemia patients. Leuk Res. 2004;28:987–90.
Chevallier P, Roland V, Mahé B, Juge-Morineau N, Dubruille V, Guillaume T, et al. Administration of mylotarg 4 days after beginning of a chemotherapy including intermediate-dose aracytin and mitoxantrone (MIDAM regimen) produces a high rate of complete hematologic remission in patients with CD33+ primary resistant or relapsed acute myeloid leukemia. Leuk Res. 2005;29:1003–7.
Stone RM, Moser B, Sanford B, Schulman P, Kolitz JE, Allen S, et al. Cancer and Leukemia Group B. High dose cytarabine plus gemtuzumab ozogamicin for patients with relapsed or refractory acute myeloid leukemia: cancer and Leukemia Group B study 19902. Leuk Res. 2011;35:329–33.
Estey EH, Thall PF, Giles FJ, Wang XM, Cortes JE, Beran M, et al. Gemtuzumab ozogamicin with or without interleukin 11 in patients 65 years of age or older with untreated acute myeloid leukemia and high-risk myelodysplastic syndrome: comparison with idarubicin plus continuous-infusion, high-dose cytosine arabinoside. Blood. 2002;99:4343–9.
De Angelo DJ, Stone RM, Durrant S, Liu D, Baccarani M, Schiffer CA, et al., Mylotarg Study Group. Gemtuzumab Ozogamicin (Mylotarg®) in combination with induction chemotherapy for the treatment of patients with de novo acute myeloid leukemia: two age-specific phase 2 trials. Blood. 2003;102:100a, Abstr. #341.
Amadori S, Suciu S, Willemze R, Mandelli F, Selleslag D, Stauder R, et al. EORTC leukemia group; GIMEMA leukemia group. Sequential administration of gemtuzumab ozogamicin and conventional chemotherapy as first line therapy in elderly patients with acute myeloid leukemia: a phase II study (AML-15) of the EORTC and GIMEMA leukemia groups. Haematologica. 2004;89:950–6.
Fianchi L, Pagano L, Leoni F, Storti S, Voso MT, Valentini CG, et al. Gemtuzumab ozogamicin, cytosine arabinoside, G-CSF combination (G-AraMy) in the treatment of elderly patients with poor-prognosis acute myeloid leukemia. Ann Oncol. 2008;19:128–34.
Petersdorf S, Kopecky K, Stuart RK, Larson RA, Nevill TJ, Stenke L, et al. Preliminary results of Southwest Oncology Group Study S0106: an international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114:Abstr.#790.
Delaunay J, Recher C, Pigneux A, Witz F, Vey N, Blanchet O, et al. Addition of Gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of aml patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR Study. Blood. 2011;118:Abstr.#79.
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–77.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–31.
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16.
Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley K. et al; UK National Cancer Research Institute AML Working Group. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27:75–81.
Reinhardt D, Diekamp S, Fleischhack G, Corbacioglu C, Jürgens H, Dworzak M, et al. Gemtuzumab ozogamicin (Mylotarg) in children with refractory or relapsed acute myeloid leukemia. Onkologie. 2004;27:269–72.
Brethon B, Yakouben K, Oudot C, Boutard P, Bruno B, Jérome C, et al. Efficacy of fractionated gemtuzumab ozogamicin combined with cytarabine in advanced childhood myeloid leukaemia. Br J Haematol. 2008;143:541–7.
Zwaan CM, Reinhardt D, Zimmerman M, Hasle H, Stary J, Stark B, et al. Salvage treatment for children with refractory first or second relapse of acute myeloid leukaemia with gemtuzumab ozogamicin: results of a phase II study. Br J Haematol. 2010;148:768–76.
Arceci RJ, Sande J, Lange B, Shannon K, Franklin J, Hutchinson R, et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106:1183–8.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Aplenc R, Alonzo TA, Gerbing RB, Lange BJ, Hurwitz CA, Wells RJ, et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:2390–3295.
Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118:761–9.
Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K, et al. Nordic Society of Paediatric Haematology and Oncology (NOPHO). Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood. 2012;120:978–84.
Takeshita A, Shinjo K, Naito K, Matsui H, Sahara N, Shigeno K, et al. Efficacy of gemtuzumab ozogamicin on ATRA- and arsenic-resistant acute promyelocytic leukemia (APL) cells. Leukemia. 2005;19:1306–11.
Asou N, Adachi K, Tamura U, Kanamaru A, Kageyama S, Hiraoka A, et al. Analysis of prognostic factorsin newly diagnosed patients with acute promyelocytic leukemia: the APL92 study of the Japan Adult Leukemia Study Group (JALSG). Cancer Chemother Pharmacol. 2001;48(Suppl 1):S65–71.
Ohno R, Asou N, Ohnishi K. Treatment of acute promyelocytic leukemia: strategy toward further increase of cure rate. Leukemia. 2003;17:1454–63.
Douer D. New advances in the treatment of acute promyelocytic leukemia. Int J Hematol. 2002;76(Suppl 2):179–87.
Tobita T, Takeshita A, Kitamura K, Ohnishi K, Yanagi M, Hiraoka A, et al. Treatment with a new synthetic retinoid, Am80, of acute promyelocytic leukemia relapsed from complete remission induced by all-trans retinoic acid. Blood. 1997;90:967–73.
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–8.
Estey EH, Giles FJ, Beran M, O’Brien S, Pierce SA, Faderl SH, et al. Experience with gemtuzumab ozogamycin (“Mylotarg”) and all-trans retinoic acid in untreated acute promyelocytic leukemia. Blood. 2002;99:4222–4.
Lo Coco F, Cimino G, Breccia M, Noguera NI, Diverio D, Finolezzi E, et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood. 2004;104:1995–9.
Takeshita A, Shinjo K, Naito K, Matsui H, Sahara N, Shigeno K, et al. Two patients with all-trans retinoic acid-resistant acute promyelocytic leukemia treated successfully with gemtuzumab ozogamicin as a single agent. Int J Hematol. 2005;82:445–8.
Aribi A, Kantarjian HM, Estey EH, Koller CA, Thomas DA, Kornblau SM, et al. Combination therapy with arsenic trioxide, all-trans retinoic acid, and gemtuzumab ozogamicin in recurrent acute promyelocytic leukemia. Cancer. 2007;109:1355–9.
Breccia M, Cimino G, Diverio D, Gentilini F, Mandelli F, Lo Coco F. Sustained molecular remission after low dose gemtuzumab-ozogamicin in elderly patients with advanced acute promyelocytic leukemia. Haematologica. 2007;92:1273–4.
Estey EH, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, Kantarjian H. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–73.
Ravandi F, Estey E, Jones D, Faderl S, O’Brien S, Fiorentino J, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–10.
Acknowledgments
I am grateful to Drs. Toshio Kitamura and Hitoshi Kiyoi for the opportunity to present this review.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takeshita, A. Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia. Int J Hematol 97, 703–716 (2013). https://doi.org/10.1007/s12185-013-1365-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-013-1365-1