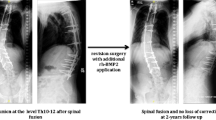
Abstract
Recombinant human bone morphogenetic protein - 2 (rh-BMP-2) was first approved by the United States Food and Drug Administration (FDA) in 2002 for use in anterior lumbar interbody fusions. Since that time, it has been estimated that “off label” use accounts for 85 % of applications. Original, industry sponsored studies demonstrated superior fusion rates with decreased incidence of complications when compared with traditional iliac crest bone graft. These studies have been criticized for potential bias and newer research has detailed potential complications as well as alternative applications. Potential off label uses of rhBMP-2 include: anterior lumbar fusions, single level posterior lumbar fusions, multiple level posterior lumbar fusions, posterior cervical fusions, long deformity fusions, in the presence of vertebral osteomyelitis, and in patients with history of malignancy. A review of the literature related to rhBMP-2 was conducted to evaluate its use for the above-mentioned applications with a special focus on fusion rates, observed complications, and clinical or radiographic outcomes.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
United States Food and Drug Administration Website.
Hsu WK, Nickoli MS, Wang JC, et al. Improving the clinical evidence of bone graft substitute technology in lumbar spine surgery. Glob Spine J. 2012;2:239–48.
Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–49.
Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–408.
Haid Jr RW, Branch Jr CL, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527–38. discussion 538–529.
Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. Represents a study critical of past research pertaining to rhBMP-2. Raises concerns of safety and side effects.
Fu R, Selph S, McDonagh M, et al. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158:890–902. Results from YODA study. Demonstrated similar clinical outcomes of rhBMP-2 and iliac crest bone graft.
Simmonds MC, Brown JV, Heirs MK, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158:877–89. Results from the Yale University Open Access Data Project (YODA). Demonstrating increased radiographic fusions, and clinically insignificant differences in patient outcomes. Results inconclusive for increased cancer risk.
Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31:775–81.
Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91:1181–9.
Tepper G, Rabbani R, Yousefzadeh M, Prince D. Quantitative assessment of retrograde ejaculation using semen analysis, comparison with a standardized qualitative questionnaire, and investigating the impact of rhBMP-2. Spine. 2013;38:841–5.
Deutsch H. High-dose bone morphogenetic protein-induced ectopic abdomen bone growth. Spine J. 2010;10:e1–4.
Lykissas MG, Aichmair A, Sama AA, et al. Nerve injury and recovery after lateral lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a cohort-controlled study. Spine J. 2014;14:217–24.
Pimenta L, Marchi L, Oliveira L, Coutinho E, Amaral R. A prospective, randomized, controlled trial comparing radiographic and clinical outcomes between stand-alone lateral interbody lumbar fusion with either silicate calcium phosphate or rh-BMP2. J Neurol Surg A Cent Eur Neurosurg. 2013;74:343–50.
Hughes AP, Taher F, Farshad M, Aichmair A. Multiple myeloma exacerbation following utilization of bone morphogenetic protein-2 in lateral lumbar interbody fusion: a case report and review of the literature. Spine J. 2014;14:e13–9.
Taher F, Lebl DR, Hughes AP, Girardi FP. Contralateral psoas seroma after transpsoas lumbar interbody fusion with bone morphogenetic protein-2 implantation. Spine J. 2013;13:e1–5.
Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–73.
Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix vs iliac crest bone graft. Spine. 2006;31:2534–9. discussion 2540.
Dimar II JR, Glassman SD, Burkus JK, Pryor PW, Hardacker JW, Carreon LY. Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am. 2009;91:1377–86.
Glassman SD, Dimar III JR, Burkus K, et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 2007;32:1693–8.
Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9:623–9.
Michielsen J, Sys J, Rigaux A, Bertrand C. The effect of recombinant human bone morphogenetic protein-2 in single-level posterior lumbar interbody arthrodesis. J Bone Joint Surg Am. 2013;95:873–80.
Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 Suppl):S86–104.
Crandall DG, Revella J, Patterson J, Huish E, Chang M, McLemore R. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease—part 1: large series diagnosis related outcomes and complications with 2- to 9-year follow-up. Spine. 2013;38:1128–36.
Crandall DG, Revella J, Patterson J, Huish E, Chang M, McLemore R. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease—part 2: BMP dosage-related complications and long-term outcomes in 509 patients. Spine. 2013;38:1137–45.
Hoffmann MF, Jones CB, Sietsema DL. Complications of rhBMP-2 utilization for posterolateral lumbar fusions requiring reoperation: a single practice, retrospective case series report. Spine J. 2013;13:1244–52.
Hamilton DK, Jones-Quaidoo SM, Sansur C, Shaffrey CI, Oskouian R, Jane Sr JA. Outcomes of bone morphogenetic protein-2 in mature adults: posterolateral non-instrument-assisted lumbar decompression and fusion. Surg Neurol. 2008;69:457–61. discussion 461–452.
Lee KB, Taghavi CE, Hsu MS, et al. The efficacy of rhBMP-2 vs autograft for posterolateral lumbar spine fusion in elderly patients. Eur Spine J. 2010;19:924–30.
Deyo RA, Ching A, Matsen L, et al. Use of bone morphogenetic proteins in spinal fusion surgery for older adults with lumbar stenosis: trends, complications, repeat surgery, and charges. Spine. 2012;37:222–30.
Hoffmann MF, Jones CB, Sietsema DL. Recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) in posterolateral lumbar spine fusion: complications in the elderly. J Orthop Surg Res. 2013;8:1.
Perri B, Cooper M, Lauryssen C, Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235–9.
Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein vs an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426–35.
Fineberg SJ, Ahmadinia K, Oglesby M, Patel AA, Singh K. Hospital outcomes and complications of anterior and posterior cervical fusion with bone morphogenetic protein. Spine. 2013;38:1304–9.
Hodges SD, Eck JC, Newton D. Retrospective study of posterior cervical fusions with rhBMP-2. Orthopedics. 2012;35:e895–8.
Dorward IG, Buchowski JM, Stoker GE, Zebala LP. Posterior cervical fusion with recombinant human bone morphogenetic protein-2: complications and fusion rate at minimum two-year follow-up. J Spinal Disord Tech. 2013. doi:10.1097/BSD.0b013e318286fa7e.
Crawford III CH, Carreon LY, McGinnis MD, Campbell MJ, Glassman SD. Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge vs iliac crest bone graft for posterior cervical arthrodesis. Spine. 2009;34:1390–4.
Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66.
Hiremath GK, Steinmetz MP, Krishnaney AA. Is it safe to use recombinant human bone morphogenetic protein in posterior cervical fusion? Spine. 2009;34:885–9.
Mulconrey DS, Bridwell KH, Flynn J, Cronen GA, Rose PS. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine. 2008;33:2153–9.
Kim HJ, Buchowski JM, Zebala LP, Dickson DD, Koester L, Bridwell KH. RhBMP-2 is superior to iliac crest bone graft for long fusions to the sacrum in adult spinal deformity: four- to 14-year follow-up. Spine. 2013;38:1209–15.
Maeda T, Buchowski JM, Kim YJ, Mishiro T, Bridwell KH. Long adult spinal deformity fusion to the sacrum using rhBMP-2 vs autogenous iliac crest bone graft. Spine. 2009;34:2205–12.
Bess SL, Line BG, Lafage V, et al. International Spine Study Group ISSG. Does recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) use in adult spinal deformity (ASD) increase complications and are complications associated with location of rhBMP-2 use?: a prospective, multicenter study of 279 consecutive patients. Spine. 2013.
Mesfin A, Buchowski JM, Zebala LP, et al. High-dose rhBMP-2 for adults: major and minor complications: a study of 502 spine cases. J Bone Joint Surg Am. 2013;95:1546–53.
Rahman RK, Buchowski JM, Stephens B, Dorward IG, Koester LA, Bridwell KH. Comparison of TLIF with rhBMP-2 versus no TLIF and higher posterolateral rhBMP-2 dose At L5-S1 for long fusions to the sacrum with sacropelvic fixation in patients with primary adult deformity. Spine. 2013;38:2264–71.
Miller CP, Simpson AK, Whang PG, et al. Effects of recombinant human bone morphogenetic protein 2 on surgical infections in a rabbit posterolateral lumbar fusion model. Am J Orthop. 2009;38:578–84.
O’Shaughnessy BA, Kuklo TR, Ondra SL. Surgical treatment of vertebral osteomyelitis with recombinant human bone morphogenetic protein-2. Spine. 2008;33:E132–9.
Allen RT, Lee YP, Stimson E, Garfin SR. Bone morphogenetic protein-2 (BMP-2) in the treatment of pyogenic vertebral osteomyelitis. Spine. 2007;32:2996–3006.
Aryan HE, Lu DC, Acosta Jr FL, Ames CP. Corpectomy followed by the placement of instrumentation with titanium cages and recombinant human bone morphogenetic protein-2 for vertebral osteomyelitis. J Neurosurg Spine. 2007;6:23–30.
Holtzhausen A, Golzio C, How T, et al. Novel bone morphogenetic protein signaling through Smad2 and Smad3 to regulate cancer progression and development. FASEB J. 2014;28:1248–67.
Kokorina NA, Zakharkin SO, Krebsbach PH, Nussenbaum B. Treatment effects of rhBMP-2 on invasiveness of oral carcinoma cell lines. Laryngoscope. 2011;121:1876–80.
Rici RE, Alcantara D, Fratini P, et al. Mesenchymal stem cells with rhBMP-2 inhibits the growth of canine osteosarcoma cells. BMC Vet Res. 2012;8:17.
Carragee EJ, Chu G, Rohatgi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95:1537–45.
Mines D, Gu Y, Kou TD, Cooper GS. Recombinant human bone morphogenetic protein-2 and pancreatic cancer: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2011;20:111–8.
Garulli C, Kalogris C, Pietrella L, et al. Dorsomorphin reverses the mesenchymal phenotype of breast cancer initiating cells by inhibition of bone morphogenetic protein signaling. Cell Signal. 2014;26:352–62.
Boeuf S, Bovee JV, Lehner B, et al. BMP and TGFbeta pathways in human central chondrosarcoma: enhanced endoglin and Smad 1 signaling in high grade tumors. BMC Cancer. 2012;12:488.
Orui H, Imaizumi S, Ogino T, Motoyama T. Effects of bone morphogenetic protein-2 on human tumor cell growth and differentiation: a preliminary report. J Orthop Sci. 2000;5:600–4.
Sotobori T, Ueda T, Myoui A, et al. Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin beta1 into lipid rafts. Exp Cell Res. 2006;312:3927–38.
Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–81.
Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7:301–7.
Hamilton DK, Smith JS, Reames DL, Williams BJ, Chernavvsky DR, Shaffrey CI. Safety, efficacy, and dosing of recombinant human bone morphogenetic protein-2 for posterior cervical and cervicothoracic instrumented fusion with a minimum 2-year follow-up. Neurosurgery. 2011;69:103–11. discussion 111.
Dagostino PR, Whitmore RG, Smith GA, Maltenfort MG, Ratliff JK. Impact of bone morphogenetic proteins on frequency of revision surgery, use of autograft bone, and total hospital charges in surgery for lumbar degenerative disease: review of the Nationwide Inpatient Sample from 2002 to 2008. Spine J. 2014;14:20–30. Examines cost and re-operation rates in patients receiving rhBMP-2. Demonstrated that BMP-2 use has led to higher hospital charges.
Compliance with Ethics Guidelines
Conflict of Interest
Brett Walker, John Koerner, and Sriram Sankarayanaryanan declare that they have no conflict of interest. Kris Radcliff has been a consultant for and has received royalties from Globus Medical, has received honoraria from Depuy, and has received travel/accommodations expenses covered or reimbursed from Depuy, Globus, Medtronic, Relievant, and Stryker.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, B., Koerner, J., Sankarayanaryanan, S. et al. A consensus statement regarding the utilization of BMP in spine surgery. Curr Rev Musculoskelet Med 7, 208–219 (2014). https://doi.org/10.1007/s12178-014-9224-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-014-9224-0