Abstract
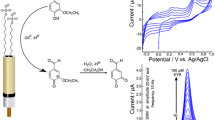
Cyclic and square wave voltammetric measurements were performed to deduce the electrochemical behavior of fomesafen herbicide on the prepared carbon nanotube paste electrodes. Fomesafen has created a well-defined cathodic peak at −540 mV (vs. Ag/AgCl), but no corresponding oxidation peak has appeared on the reverse scan. The influence of the pH on the electro-reduction peak was tested at various pH values, and the E p versus pH plot suggested that protons are involved in reduction process. Electrochemical studies showed that –NO2 group was responsible for the reduction process. A linear relationship has been constructed in the concentration range of 0.30–40 mg/L. The limits of detection and quantification values were obtained as 0.089 and 0.297 mg/L, respectively. Fomesafen was determined in the presence of some well-known pesticides, and the extent of recoveries of 5 mg/L fomesafen in the presence of equal amounts of pesticides anilazine, pymetrozine, and triflumizole was 103.7 ± 0.9, 94.3 ± 0.4, and 97.9 ± 0.5 %, respectively (n = 3). The accuracy of the recommended method was further proved by the determination of fomesafen in spiked real samples such as apricot juice, cherry juice, and lake water with a relative error of −4.2, −2.8, and −1.8 %, respectively. The obtained results suggest that the recommended method is sufficiently accurate, selective, and precise.







Similar content being viewed by others
References
Barek J, Cabalkova D, Fischer J, Navratil T, Peckova K, Yosypchuk B (2011) Voltammetric determination of the herbicide bifenox in drinking and river water using a silver solid amalgam electrode. Environ Chem Lett 9:83–86
Demir E, Kemer B, Isildak I, Aboul-Enein HY (2015) Fe3+-ion selective electrode developed as a detector in flow injection analysis. Curr Anal Chem 11:104–108
Goyal RN, Gupta VK, Bachheti N (2007) Fullerene-C60-modified electrode as a sensitive voltammetric sensor for detection of nandrolone—an anabolic steroid used in doping. Anal Chim Acta 597:82–89
Goyal RN, Gupta VK, Chatterjee S (2010) Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sensors Actuators B 149:252–258
Guiberteaua A, Galeanoa T, Moraa N, Parrillab P, Salinasa F (2001) Study and determination of the pesticide imidacloprid by square wave adsorptive stripping voltammetry. Talanta 53:943–949
Gupta VK, Jain S, Khurana (1997a) A PVC-based pentathia-15-crown-5 membrane potentiometric sensor for mercury (II). Electroanalysis 9:478–480
Gupta VK, Jain AK, Singh LP, Khurana U (1997b) Porphyrins as carrier in PVC based membrane potentiometric sensors for nickel(II). Anal Chim Acta 355:33–41
Gupta VK, Mangla R, Khurana U, Kumar P (1999) Determination of uranyl ions using poly(vinyl chloride) based 4-tert-butylcalix[6]arene membrane sensor. Electroanalysis 11:573–576
Gupta VK, Prasad R, Kumar P, Mangla R (2000) New nickel(II) selective potentiometric sensor based on 5,7,12,14 tetramethyldibenzotetraazaannulene in a poly(vinyl chloride) matrix. Anal Chim Acta 420:19–27
Gupta VK, Chandra S, Mangla R (2002) Dicyclohexano-18-crown-6 as active material in PVC matrix membrane for the fabrication of cadmium selective potentiometric sensor. Electrochim Acta 47:1579–1586
Gupta VK, Jain S, Chandra S (2003) Chemical sensor for lanthanum(III) determination using aza-crown as ionophore in poly(vinyl chloride) matrix. Anal Chim Acta 486:199–207
Gupta VK, Jain AK, Maheshwari G, Lang H, Ishtaiwi Z (2006) Copper(II)-selective potentiometric sensors based on porphyrins in PVC matrix. Sensors Actuators B 117:90–106
Gupta VK, Jain AK, Maheshwari G (2007a) Aluminum(III) selective potentiometric sensor based on morin in poly(vinyl chloride) matrix. Talanta 72:1469–1473
Gupta VK, Singh AK, Al Khayat M, Gupta B (2007b) Neutral carriers based polymeric membrane electrodes for selective determination of mercury (II). Anal Chim Acta 590:81–90
Gupta VK, Goyal RN, Sharma RA (2008) Anion recognition using newly synthesized hydrogen bonding disubstituted phenylhydrazone-based receptors. Poly(vinyl chloride)-based sensor for acetate. Talanta 76:859–864
Gupta VK, Arunima N, Singhaj B, Agarwal S (2011a) Recent advances on potentiometric membrane sensors for pharmaceutical analysis. Comb Chem High Throughput Screen 14:284–302
Gupta VK, Ganjali MR, Norouzi P, Khani H, Nayak A, Agarwal S (2011b) Electrochemical analysis of some toxic metals by ion–selective electrodes. Crit Rev Anal Chem 41:282–313
Gupta VK, Singh LP, Singh R, Upadhyay N, Kaur SP, Sethi B (2012) A novel copper (II) selective sensor based on dimethyl 4, 4′ (o-phenylene) bis(3-thioallophanate) in PVC. J Mol Liq 174:11–16
Gupta VK, Sethi B, Sharma RA, Agarwal S, Bharti A (2013) Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J Mol Liq 177:114–118
Gupta VK, Singh AK, Lokesh KK (2014) Thiazole Schiff base turn-on fluorescent chemosensor for Al3+ ion. Sensors Actuators B195:98–108
Gupta VK, Karimi-Maleh H, Sadegh R (2015a) Simultaneous determination of hydroxylamine, phenol and sulfite in water and waste water samples using a voltammetric nanosensor. Int J Electrochem Sci 10:303–316
Gupta VK, Mergu N, Lokesh KK, Singh AK (2015b) A reversible fluorescence “off–on–off” sensor for sequential detection of aluminum and acetate/fluoride ions. Talanta 144:80–89
Hayashi Y, Kouji H (1990) Synthesis and herbicidal activity of geometrical isomers of methyl[[[1-[5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrophenyl]-2 methoxyethylidene]amino]oxy]acetate. J Agric Food Chem 38:845–850
Higgins JM, Whitwell T, Murdock EC, Toler JE (1988) Recovery of pitted morningglory (ipomoea-lacunosa) and ivyleaf morningglory (ipomoea-hederacea) following applications of acifluorfen, fomesafen, and lactofen. Weed Sci 36:345–353
Hong-Li S, Yu-Hsiang S, Mahaveer MB, Shang-Da H (2006) Determination of diphenylether herbicides in water samples by solid-phase microextraction coupled to liquid chromatography. J Sep Sci 29:2647–2652
İnam R, Çakmak Z (2013) A simple square wave voltammetric method for the determination of aclonifen herbicide. Anal Meth 5:3314–3320
İnam R, Caykara T, Kantoglu O (2003) Polarographic determination of uranyl adsorption onto poly(acrylamide-g-ethylenediaminetetraacetic acid) hydrogels in the presence of cadmium and lead. Nucl Instrum Meth B 208:400–404
Jain AK, Gupta VK, Singh LP, Raisoni JR (2006) Comparative study of Pb2+ selective sensors based on derivatized tetrapyrazole and calix[4]arene receptors. Electro Chim Acta 51:2547–2553
Jain R, Gupta VK, Jadon N, Radhapyari K (2010) Voltammetric determination of cefixime in pharmaceuticals and biological fluids. Anal Biochem 407:79–88
JFCRF (2015) The Japan Food Chemical Research Foundation, http://www.m5.ws001.squarestart.ne.jp/foundation/agrdtl.php?a_inq=72200
Khani H, Rofouei MK, Arab P, Gupta VK, Vafaei Z (2010) Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: application to potentiometric monitoring of mercury ion(II). J Hazard Mater 183:402–409
Kotoucek M, Opravilova M (1996) Voltammetric behaviour of some nitropesticides at the mercury drop electrode. Anal Chim Acta 329:73–81
Lagana A, Fago G, Fasciani L, Marino A, Mosso M (2000) Determination of diphenyl-ether herbicides and metabolites in natural waters using high-performance liquid chromatography with diode array tandem mass spectrometric detection. Anal Chim Acta 414:79–94
Laviron E, Roullier L, Degrand C (1980) A multilayer model for the study of space distributed redox modified electrodes: part II. Theory and application of linear potential sweep voltammetry for a simple reaction. J Electroanal Chem 112:11–23
Lovric M, Komorsky-Lovric S (1988) Square-wave voltammetry of an adsorbed reactant. J Electroanal Chem 248:239–253
Mercan H, Inam R (2008) Determination of the fungicide anilazine in soil and river water by differential pulse polarography. Clean-Soil Air Water 36:913–919
Miller JC, Miller JN (1988) Statistics for analytical chemistry, 2nd edn. John Wiley and Sons, New York
Norouzi P, Gupta VK, Asif M, Rasoolipour S, Faridbod F, Ganjali MR (2014) Determination of methyl parathion in liquid phase by nano-composite carbon paste surface biosensor and differential FFT continuous linear sweep voltammetry. J Mol Liq 198:239–245
PMRL(2014), Proposed maximum residue limit, Health Canada, http://www.hc-sc.gc.ca/cps-spc/alt_formats/pdf/pest/part/consultations/_pmrl2014-40/pmrl2014-40-eng.pdf
Prasad R, Gupta VK, Kumar A (2004) Metallo-tetraazaporphyrin based anion sensors: regulation of sensor characteristics through central metal ion coordination. Anal Chim Acta 508:61–70
Rupp E, Zhong Q, Zuman P (1992) Polarographic determination of some pesticides containing nitro group: application to a study of adsorption on lignin. Electroanal 4:11–18
Sarıgül T, İnam R (2009a) A direct method for the polarographic determination of herbicide triasulfuron and application to natural samples and agrochemical formulation. Bioelectrochem 75:55–60
Sarıgül T, İnam R (2009b) Study and determination of the herbicide cyclosulfamuron by square wave stripping voltammetry. Electrochim Acta 54:5376–5380
Srivastava SK, Gupta VK, Jain S (1995) Determination of lead using a poly(vinyl chloride)-based crown ether membrane. Analyst 120:495–498
Tseng SH, Lee WC, Chang PC, Chou SS (1988) Analytical methods for the determination of acifluorfen and bentazone residues in crops. J Food Drug Anal 6:559–572
Vencill WK (2002) Herbicide handbook, eighth edn. Weed Science Society of America, Lawrence
Zuman P (1997) Half a century of research using polarography. Microchem J 57:4–51
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by the university research grant or a company.
Conflict of Interest
Recai İnam declares that he has no conflict of interest. Ersin Demir declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Demir, E., İnam, R. Square Wave Voltammetric Determination of Fomesafen Herbicide Using Modified Nanostructure Carbon Paste Electrode as a Sensor and Application to Food Samples. Food Anal. Methods 10, 74–82 (2017). https://doi.org/10.1007/s12161-016-0551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0551-1